Acquired the world's first recombinant protein anthrax vaccine product license...Successful Localization
Apr 08, 2025
|
The Ministry of Food and Drug Safety said that the genetically modified anthrax vaccine applied by Green Cross Co., Ltd. is a "baritrax" (adsorption anthrax vaccine (genetic recombination)It said it granted ' on the 8th.
To induce the production of antibodies that prevent infection caused by anthrax, 'Verythraxju' is a vaccine designed to prevent the exposure of anthrax-induced infections in adults using gene recombination technology.
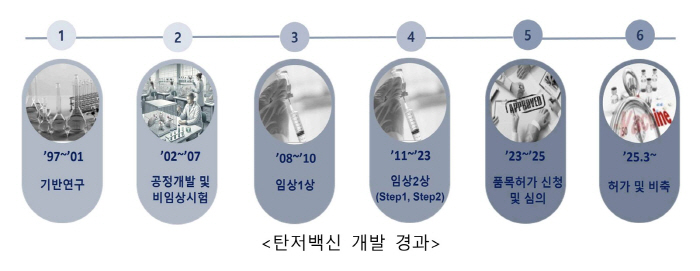
In order to prepare for public health crises such as bioterrorism, the Korea Centers for Disease Control and Prevention conducted basic research for vaccine development, starting with the discovery of anthrax vaccine candidates in 1997, and applied for drug product approval in October 2023 by conducting vaccine process development and clinical trials with Green Cross Co., Ltd. and obtained final product approval through strict deliberation procedures.
The approved anthrax vaccine is a safer vaccine that improves problems such as the possibility of side effects caused by a small amount of residual anthrax toxin factors that can appear in existing commercialized vaccines, with the main component being the Protective Antigen (PA) protein. It was organized by the Korea Centers for Disease Control and Prevention and developed in cooperation with Green Cross Co., Ltd., and is the first case in the world to commercialize a anthrax vaccine using genetic recombination technology as a drug.
The Korea Centers for Disease Control and Prevention said that with the approval of the anthrax vaccine developed in Korea, the cost of importing the vaccine can be significantly reduced by replacing the anthrax vaccine, which was previously entirely dependent on imports, with a self-sufficient vaccine in Korea. In addition, in the event of an emergency such as bioterrorism, sufficient supplies can be immediately produced and secured, contributing to the establishment of national vaccine sovereignty through stable vaccine supply, he added.
Clinical trials in healthy adults confirmed that antibodies capable of neutralizing the toxin of B. anthracis were significantly produced in the anthrax vaccination group, and no cases of acute or severe abnormalities occurred. It was confirmed that there was no difference in minor abnormalities between the vaccination group and the placebo group, confirming the effectiveness and safety of the vaccine.
Director of the Korea Centers for Disease Control and Prevention Ji Young-mi said, "Having a vaccine developed in Korea to respond to infectious diseases of bioterrorism is very important in preparing for bioterrorism. The localization of low-carbon vaccines is expected to enable rapid and active responses in the event of a national public health crisis such as bioterrorism, and contribute greatly to strengthening global health security beyond responding to infectious diseases."," he said.
This article was translated by Naver AI translator.