Chronic inflammation such as rheumatoid and psoriasis and suppresses by controlling gene switches
Aug 26, 2025
|
Chronic inflammatory diseases such as rheumatoid arthritis, psoriasis, and sepsis worsen symptoms due to excessive activation of immune cells and excessive secretion of inflammatory proteins called the tumor necrosis factor alpha (TNFα)'. For treatment, antibody treatments that block proteins are used, but they are expensive and some patients do not respond or have limitations such as risk of infection.
Professor Kim Rak-kyun of Yonsei University's Medical Life Sciences Department, Dr. Kim Soo-min (doctor scientist), and Dr. Cho Min-jeong's research team conducted an international joint study with Professor Richard Flavel of Yale University School of Medicine in the United States. The research team paid attention to the Super-Enhancer and its transcription product, eRNA. Super-incenters and eRNAs are molecular switches that control the expression of specific genes and are attracting attention as selective treatment targets because they are only activated during diseases.
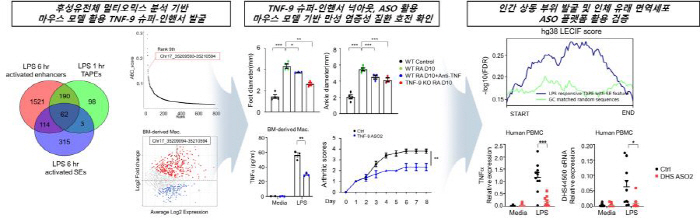
The research team found the TNF-9 Super-Inhancer, a key genetic switch that regulates TNFα in mouse immune cells. To this end, we identified 62 eRNA-generated super-inhandsers that regulate gene activation using advanced dielectric analysis technology. Among them, TNF-9 eRNA, a key super-inhander of TNFα, was first selected as a target. When this area was removed or the eRNA produced there was suppressed, TNFα decreased and arthritis and psoriasis symptoms in mice were alleviated.
The same results were obtained in experiments with patient-derived cells.
The research team confirmed that the DHS44500 Super-Inhancer, which corresponds to TNF-9 of mice in humans, is active in immune cells of rheumatoid patients. When DHS44500 eRNA was suppressed in the patient's blood immune cells using anti-sense oligonucleotide (ASO), TNFα expression was significantly reduced and the inflammatory response was also calmed.
ASO, which was used in the study, is a next-generation therapeutic platform that regulates the expression of specific RNAs. Spinraza, a treatment for spinal muscular dystrophy, is a representative ASO-based new drug. In this study, we confirmed that by precisely inhibiting TNF-9 eRNA, which is inflammation-specifically activated through ASO, only pathological inflammation can be selectively blocked while preserving normal immune function.
Professor Kim Rak-gyun, who led the study, said "This study is the first to precisely inhibit TNFα expression by directly targeting super-inhandser-derived eRNAs with ASO. Existing antibody treatments indiscriminately blocked the entire TNFα protein, which was followed by a risk of infection. We hope that this study will serve as the basis for the development of next-generation chronic inflammatory treatments that have reduced side effects and increased treatment effectiveness."
The study was recently published in the international journal 『Advanced Science』.
|
This article was translated by Naver AI translator.