Global Big Pharma is participating in the development of radioactive drugs that complement the limitations of existing anticancer drugs...Best Time for Korean Companies to Seek Global Expansion
Sep 29, 2025
|
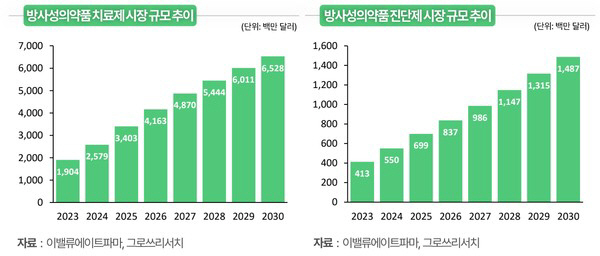
Groth Research published a report on the 29th that focused on the industrial significance of radioactive drugs that extend beyond prostate cancer and neuroendocrine tumors to solid cancers such as lung cancer, breast cancer, and melanoma and neurological diseases such as Alzheimer's and Parkinson's disease.
Radioactive drugs are a new therapeutic modality that complements the limitations of existing anticancer drugs by combining radioactive isotopes and target substances to deliver radiation directly to tumors or specific tissues. PluVicto (Novartis, Prostate Cancer) and Rutatera (Neuroendocrine Tumor) have already been commercialized, demonstrating extended survival, and showing a large investment in global Big Pharma.
In particular, radioactive ligand treatment (RLT) is a selective treatment that only hits cancer cells precisely, and has become a new alternative in elderly patients and treatment-resistant patients while significantly reducing side effects compared to existing anticancer drugs. Novartis' Pluvicto grew more than 30% year-on-year in the second quarter of 2025 to $450 million in revenue, while Rutatera also secured a stable market as a treatment for rare cancers.
|
Changes are also evident in the global competition. With Novartis leading the commercialization, Bayer (Xofigo), BMS (acquisition of RayzeBio), AstraZeneca (acquisition of Fusion Pharma), and Eli Lilly (acquisition of POINT Biopharma) entered the market one after another. In the supply chain, a small number of companies exclusively supply key isotopes such as Lu-177 and Ac-225 and form a long-term contract structure.
In Korea, Future Chem, Cellvion, DuChem Bio, and SK Biopharmaceuticals are entering the market in earnest. Futurechem has been approved for phase 3 clinical trials of FC705, a candidate for prostate cancer treatment, and Selvion is pursuing a differentiation strategy through global combination clinical trials. Duchembio is securing stable sales as a diagnosis of dementia and prostate cancer, and SK Biopharmaceuticals is preparing for the phase 1 clinical trial of its new drug candidate SKL35501.
Han Yong-hee, CEO of Groth Research, evaluated that "radioactive drugs are a key platform for precision treatment that goes beyond the limits of existing anticancer drugs to cover cancer and neurological diseases. The pipelines secured by domestic companies have sufficient potential to compete with global big pharma." He then analyzed that it is the best time for domestic companies to seek global expansion.
This article was translated by Naver AI translator.