GLP1 type obesity treatment, prohibited during pregnancy and breastfeeding...Food and Drug Administration Releases Safety Use Guide
Sep 29, 2025
The GLP-1 series of obesity treatments, a recent craze, and related authorities have released safe use guides.
GLP-1 obesity treatment is a drug that has the effect of reducing appetite by imitating the action of GLP-1 (Glucagon-like peptide 1) hormones and reducing weight by maintaining a long sense of satiety.
On the 29th, the Ministry of Food and Drug Safety and the Korea Institute of Drug Safety distributed a guide to the safety of GLP-1 obesity treatments to local doctors and regional drug safety centers nationwide. This guide contains information such as ▲ diseases using obesity treatment ▲ correct administration method ▲ storage and disposal method ▲ precautions for administration ▲ adverse reactions (side effects) reporting method.
GLP-1 family obesity treatments are prescribed to obese patients with ▲early body mass index (BMI) of 30kg/m2 or more, or overweight patients with ▲ more than one weight-related comorbidities (type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) with a BMI of 27kg/m2 or more and less than 30kg/m2.
If patients taking diabetes drugs use GLP-1-based obesity drugs in combination, the risk of lower blood sugar levels may increase, so they should consult with medical staff on whether to control the dose of the drug. In addition, the use of obesity treatment is prohibited during pregnancy and lactation, and it is recommended to plan pregnancy in consideration of the drug's residual period in the body. Depending on the type of obesity treatment, contraception is required for at least one to two months after stopping the drug.
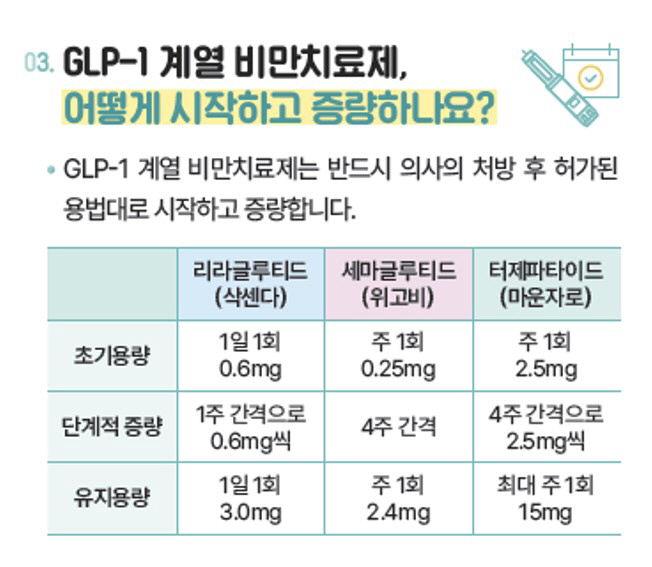
Rather than starting with a high dose from the beginning, the administration should be started and increased according to the approved usage after the doctor's prescription, and the administration method and dose should be observed according to the doctor's prescription and pharmacist's guidance. When administering obesity drugs, inject them into comfortable areas of the abdomen, thigh, or upper arm, and change the injection site each time.
In addition, it is important for patients to inform their medical professionals of their drug hypersensitivity, medications currently being administered, medical history, pregnancy, and breastfeeding prior to dosing.
Obesity treatments should be refrigerated away from light, and discarded without use if the medicine is frozen, particles are visible, or color has changed.
However, even if obesity treatment is used within the permitted range, abnormal cases such as gastrointestinal disorders, injection site reactions, fatigue, and dizziness can occur, and clinically important abnormal cases such as hypersensitivity, acute pancreatitis, cholelithiasis, and cholecystitis can also occur, so medical staff should be notified or visited.
The Ministry of Food and Drug Safety urged the use of GLP-1-based obesity treatment, which is a specialty drug, by following the pharmacist's preparation and medication guidance after a doctor's prescription and safe use of obesity treatment within the permitted range. He also emphasized that purchasing or distributing through overseas direct purchase or personal sales online should be avoided because the safety of the product cannot be guaranteed.
GLP-1 obesity treatment is a drug that has the effect of reducing appetite by imitating the action of GLP-1 (Glucagon-like peptide 1) hormones and reducing weight by maintaining a long sense of satiety.
On the 29th, the Ministry of Food and Drug Safety and the Korea Institute of Drug Safety distributed a guide to the safety of GLP-1 obesity treatments to local doctors and regional drug safety centers nationwide. This guide contains information such as ▲ diseases using obesity treatment ▲ correct administration method ▲ storage and disposal method ▲ precautions for administration ▲ adverse reactions (side effects) reporting method.
GLP-1 family obesity treatments are prescribed to obese patients with ▲early body mass index (BMI) of 30kg/m2 or more, or overweight patients with ▲ more than one weight-related comorbidities (type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) with a BMI of 27kg/m2 or more and less than 30kg/m2.
If patients taking diabetes drugs use GLP-1-based obesity drugs in combination, the risk of lower blood sugar levels may increase, so they should consult with medical staff on whether to control the dose of the drug. In addition, the use of obesity treatment is prohibited during pregnancy and lactation, and it is recommended to plan pregnancy in consideration of the drug's residual period in the body. Depending on the type of obesity treatment, contraception is required for at least one to two months after stopping the drug.
|
In addition, it is important for patients to inform their medical professionals of their drug hypersensitivity, medications currently being administered, medical history, pregnancy, and breastfeeding prior to dosing.
Obesity treatments should be refrigerated away from light, and discarded without use if the medicine is frozen, particles are visible, or color has changed.
|
The Ministry of Food and Drug Safety urged the use of GLP-1-based obesity treatment, which is a specialty drug, by following the pharmacist's preparation and medication guidance after a doctor's prescription and safe use of obesity treatment within the permitted range. He also emphasized that purchasing or distributing through overseas direct purchase or personal sales online should be avoided because the safety of the product cannot be guaranteed.
This article was translated by Naver AI translator.