A total of 1,116,694 prescriptions for Saxenda and Hugo Bee in 5 years...1,708 abnormal cases reported
|
According to data confirmed by Seo Mi-hwa (Democratic Party of Korea) of the National Assembly's Health and Welfare Committee through the Health Insurance Review and Assessment Service, the number of prescriptions aggregated in the Drug Safety and Use Service (DUR) system from January 2020 to June 2025 was 721,310 cases and 395,384 cases of Hugo.
In terms of gender, women accounted for 71.5%, far more than men, and by age group, those in their 30s and 40s accounted for about 60% of the total. By region, it was concentrated in the metropolitan areas such as Seoul (40.2 percent) and Gyeonggi (23.5 percent). Saxenda began selling in March 2018 and Hugo Bee in October 2024, and demand among the general public increased sharply as celebrities' successful diet cases became known.
The problem is that GLP-1-based obesity drug injections are prescribed for cosmetic purposes to adult obese patients with a BMI of 30kg/㎡ or more than 27kg/㎡ or less than 30kg/㎡ with one or more weight-related comorbidities such as high blood pressure. In particular, it is pointed out that the BMI verification process is sloppy.
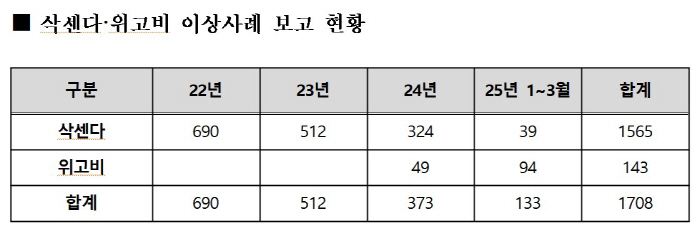
Concerns about abnormal cases are also growing. According to data from the Ministry of Food and Drug Safety (KFDA), a total of 1,708 abnormal cases were reported from 2022 to March 2025 (Saxenda 1,565 cases and Wigobi 143 cases). The main symptoms were ▲zone (404), ▲guto (168), ▲ headache (161), ▲ injection site pruritus (149), ▲ injection site rash (142), ▲ diarrhea (15), and ▲ indigestion (9 cases). Although the direct causal relationship has not been clearly proven, it is pointed out that it should be careful about taking it.
Representative Seo Mi-hwa pointed out, "'Wigobee Diet' is spreading like a trend on social media and media recently, but if it is used for cosmetic purposes by non-obese patients, it can cause serious side effects.' Even if it is an unpaid prescription drug, BMI verification should be thoroughly conducted and management and supervision should be strengthened to prevent illegal and inappropriate prescriptions," he stressed.
Recently, the Ministry of Food and Drug Safety also said that only patients with obesity should be used carefully according to the approved usage according to the prescription of medical experts.
Even if the obesity treatment is used within the permitted range, gastrointestinal adverse reactions (nausea, vomiting, diarrhea, constipation) and injection site reactions (rashes, pain, swelling, etc.) are common, and side effects such as hypersensitivity, hypoglycemia, acute pancreatitis, cholelithiasis, and fluid reduction can occur.
In addition, some drugs are prohibited from administration if they have underlying diseases such as thyroid medullary cancer, so be sure to consult with a specialist, and patients with a related medical history should be administered especially carefully because hypoglycemia and retinopathy may occur in diabetic patients (type 2).
This article was translated by Naver AI translator.