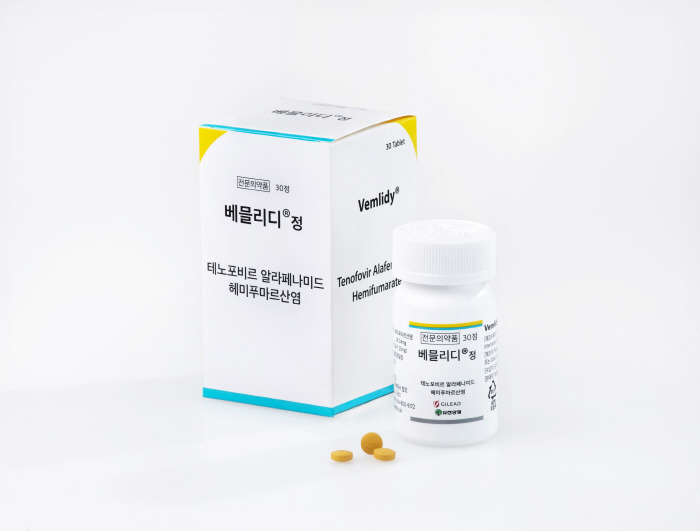
Bemlidi, a treatment for chronic hepatitis B, expanded health insurance coverage standards...Apply to a wide range of patient populations
Nov 05, 2025
|
According to the Ministry of Health and Welfare's notice of revision of the standards for applying medical care benefits, Bemlidi can be used as an initial treatment for HBV-DNA-positive patients with ▲ decompensated cirrhosis or hepatocellular carcinoma, and ▲ patients who received liver transplantation during chronic hepatitis B treatment can be continuously administered without any conditions. In addition, care benefits are recognized when administered as a preventive therapy for the risk of reactivation of ▲ chronic hepatitis B or to pregnant women with HBV-DNA of 200,000 IU/mL or more as a preventive therapy for ▲ chronic hepatitis B vertical infection, and can be used alone or as a combination therapy regardless of whether various drugs, including polypharmaceuticals, are resistant.
This is a significant relief from the existing benefit conditions, which were limited to patients with reduced renal function or osteoporosis associated with patients', resulting in Bemlidi being a tenofovir formulation covering all treatment stages from initial treatment to decompensated cirrhosis, liver transplantation, reactivation prevention, pregnant women, and resistance management.
According to Gilead Sciences Korea, Bemlidi is a targeted prodrug for tenofovir and is a treatment developed to efficiently deliver medicinal ingredients to hepatocytes with only about a tenth (25 mg) dose compared to the existing tenofovir disoproxyl fumarate (TDF). Lowering tenofovir concentration in plasma by 89%, it reduces systemic exposure while maintaining non-thermal antiviral effects on TDF, and long-term clinical trials have confirmed improvements in kidney and bone density safety compared to TDF. It is administered orally once a day regardless of whether it is eaten or not, and even if there is a renal or liver disorder, it can be administered without dose control and can be used from children over 6 years of age and 25kg of weight to adults.
Kwon-Hee Kwon, Vice President of Gilead Korea's Virus Disease Division, commented "With this expansion of pay standards, Bemlidi has been recognized as a standard treatment in seven years since its launch in Korea in 2017, which is based on the clinical benefits of TAF drugs, which were also reflected through recent global guideline updates." "Gilead continues to strive to improve the early treatment and long-term treatment environment for chronic hepatitis B based on its R&D experience accumulated over 30 years in the field of antiviral treatment. Through this expansion of benefits, we expect more patients to benefit from early antiviral treatment in Korea."," he said.
This article was translated by Naver AI translator.