Gastrointestinal drug rebamipide, dopamine nerve cell protection...Possibility of Use in Treatment of Parkinson's Disease
Jun 25, 2025
|
The Korea Institute of Oriental Medicine announced that the research results of Dr. Park Geon-hyuk and Lim Hye-sun's research team at the Institute of Oriental Medicine were published on May 17, 2025 in the international renowned journal 『Journal of Neuroinflamination" (IF 10.1, top 5% of the JCR Neurosciences category as of 2025). The first author is Dr. Lim Hye-sun, and the corresponding author is Dr. Park Geon-hyuk.
Parkinson's disease is a typical neurodegenerative disease that causes motor disorders as dopaminergic neurons gradually disappear, and fundamental treatments are limited.
In particular, many patients with Parkinson's disease are accompanied by gastrointestinal abnormalities such as constipation and gastropathy, and a change in the treatment paradigm is required in that the functions of the intestine and brain are closely connected to each other.
In oriental medicine, 'Bi(脾)', which oversees gastrointestinal function, is considered to be the main 思 of mental activity(思), and gastrointestinal and brain functions are interpreted as an interactive physiological system, not disconnected.
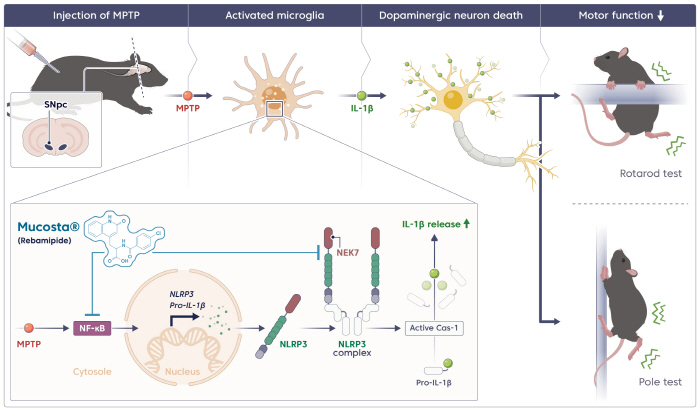
Based on this traditional theory, the researchers tested the hypothesis that rebamipide's gastrointestinal protection effect could also be applied to nerve cell protection to improve motor disorders in Parkinson's disease models. Rebamipide, a gastrointestinal drug that protects the gastrointestinal mucosa and has antioxidant and anti-inflammatory properties, has been used to treat gastritis and gastric ulcers.
According to the results of the study, rebamipide increased the survival rate of dopamine neurons by about 2.1 times and dopamine secretion by about 1.4 times in Parkinson's disease-induced experimental animals, and at the same time, the mechanism of action that regulates brain inflammation pathways by inhibiting the formation of NLRP3 and NEK7 protein complexes was also identified.
Rebamipide inhibited complex binding by blocking hydrogen bonds and halogen interactions between the two proteins, which also reduced the expression of inflammatory agents by approximately 3.7-fold. In addition, virtual docking analysis also confirmed the structural possibility that rebamipide binds to both proteins simultaneously, destabilizing the complex.
In addition, in mice in which the NLRP3 gene was suppressed by CRISPR gene editing technology, rebamipide's neuronal protection effect was reduced, proving that this inflammatory pathway is a key mechanism.
The research team said, "This study is a result of convergence research that presents a new approach to the field of modern neuroscience based on the traditional theory of oriental medicine. This is significant in that not only oriental medicine but also existing oriental medicine can be reinterpreted to expand the scope of treatment."
This article was translated by Naver AI translator.