PTSD that is clear over time...Researchers in Korea Find Why Fear Memories Don't Go Away
|
Lee Chang-joon, head of the Cognitive and Social Research Group at the Institute of Basic Science (IBS), identified the pathological mechanism of PTSD, where fear memories do not disappear, along with a research team led by Ryu In-kyun, chair professor of the Institute of Brain Convergence Science at Ewha Womans University, and proposed Gamma-Aminobutyric Acid (GABA), an inhibitory neurotransmitter made by Astrocyte, a non-neuronal cell in the brain, as a new treatment target.
The results of this study were published online in Nature Portfolio's most prestigious journal in biochemistry and molecular biology 'Signal Transduction and Targeted Therapy'.
The research team explained the background of the study that PTSD treatment currently uses antidepressants that control serotonin receptors, but only 20-30% of patients show effectiveness and the rate of treatment response is very slow, so a new PTSD treatment strategy is urgently needed.
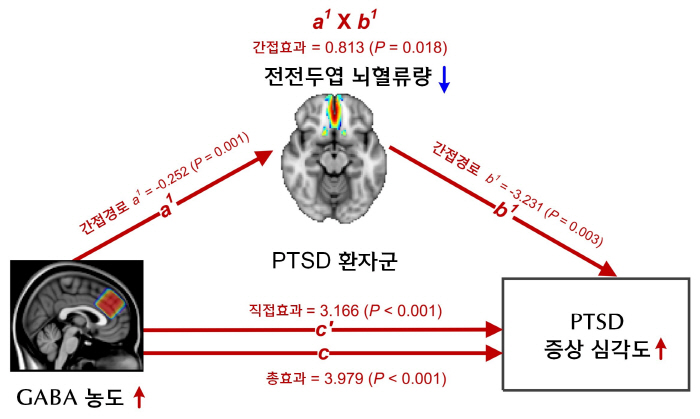
The researchers analyzed large-scale brain image data of 380 people, including PTSD patients, trauma experienced people, and the general public, and found that PTSD patients had abnormal increases in the concentration of inhibitory neurotransmitter Gabba in the prefrontal Cortex and decreased cerebral blood flow. These changes were closely related to the degree of PTSD symptoms, and in patients whose symptoms recovered, both gava concentration and cerebral blood flow were normalized.
Lee Chang-joon, head of IBS, found in a previous study that star cells, which are star-shaped non-nerve cells in the brain, produce Gabba through an enzyme called Mao B (MAOB). Based on the results of clinical brain imaging analysis, this study revealed that the deterioration of the prefrontal function in PTSD results from excessive accumulation of Gabba by star cells.
As a result of analyzing the prefrontal brain tissue of PTSD patients after the death, it was found that Mao B's activity in star cells was abnormally increased and reactive astrocytes in brain tissue were expanded. At the same time, the expression of the Gabba-degrading enzyme (ABAT) decreased, resulting in pathological changes in the overproduction and accumulation of Gabba.
In animal experiments to determine the relationship with the deterioration of the prefrontal function, the PTSD animal model, which increased the Mao B activity of star cells, showed a significant decline in the brain's recovery function, which lasted a long time and reduced fear memory. Conversely, when Mao B's activity was suppressed and returned to normal levels, this reaction was alleviated. This result is an explanation that the accumulation of Gabba following Maobi hyperactivity of star cells proves to be the cause of persistence of fear memory in PTSD.
Researchers confirmed the effectiveness of administering a new drug candidate 'KDS2010' that selectively inhibits the Maobie enzyme to the PTSD animal model, and found that star cells' Gabba concentration and brain blood flow were restored to normal levels, and anxiety behavior symptoms were alleviated. Developed from basic research by Lee Chang-joon, the new drug candidate has completed safety verification and is currently in phase 2 clinical trials.
Lee Chang-joon, head of IBS, opened the possibility of fundamental treatment of PTSD by identifying the pathologic mechanism of PTSD at the molecular and cell level and presenting a new therapeutic target called Dagassifo"We expect it to lead to the development of new mental disease treatment strategies through cell regulation by star cell regulation."
Ryu In-kyun, chair professor at Ewha Womans University, said, "This study is a representative case of Reverse Translational Research, which starts with clinical clues, confirms mechanisms in animal models, and extends to the verification of the effectiveness of new drugs. It presents a new path for mental disease treatment research with an approach that integrates clinical and basic research."," he said.
This article was translated by Naver AI translator.