Pneumococcus 20 Introduces National Vaccination for Protein-Binding Vaccine...Inoculation starts on October 1st
Aug 04, 2025
|
The Korea Centers for Disease Control and Prevention announced on the 4th that it will administer PCV20 vaccination to children and adolescents over two months of age from October.
Pneumococcus pneumoniae, a major bacterial pathogen that causes various diseases such as otitis media, pneumonia, and meningitis in infants and young children, can cause life-threatening invasive infections (IPD), especially in children with weak immune systems, so vaccination is very important.
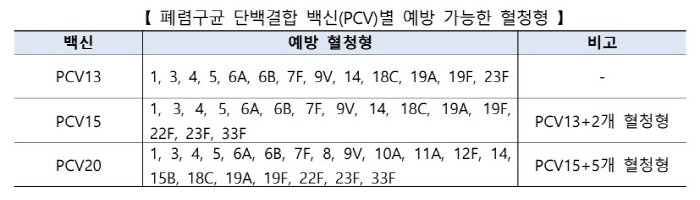
Currently, the National Vaccination Project (NIP) supports 13-valent protein-binding vaccines (hereinafter referred to as 'PCV13') and 15-valent protein-binding vaccines (hereinafter referred to as 'PCV15') for childhood pneumocococcal vaccinations. PCV20, which was approved by the Ministry of Food and Drug Safety in October last year, was decided to be introduced by the vaccination committee after comprehensively reviewing the safety, immunogenicity, and cost-effectiveness of the vaccine.
In addition to the 15 serotypes included in the existing 15-valent vaccine (PCV15), PCV20 includes an additional 5 types (8, 10A, 11A, 12F, and 15B), allowing a total of 20 types of pneumococcal serotypes to be prevented. According to this introduction, there are three types of pneumococcal vaccines that can be supported by the national vaccination project: PCV13, PCV15, and PCV20.
The inoculation schedule for healthy children is the same as before, with a total of three inoculations at 2, 4, and 6 months of age and one additional inoculation every 12 to 15 months, and children who have already started inoculation with PCV13 can be cross-vaccinated with PCV20. However, if inoculation with PCV15 is started, it is recommended to complete the vaccination with the same vaccine.
In addition, high-risk children and adolescents who are vulnerable to infection due to decreased immunity, chronic diseases, and artificial cow transplantation can be inoculated with PCV20. In the case of high-risk children and adolescents, the vaccination schedule differs depending on their age at the time of vaccination and their existing vaccination history, so they should follow the vaccination schedule appropriate to the vaccination situation.
Meanwhile, the age limit for high-risk children who can receive PCV20 will also be raised from 12 to 18.
Director of the Korea Centers for Disease Control and Prevention Lim Seung-kwan said, "With the introduction of this new PCV20 vaccine, our children can be protected from more pneumococcal serotypes. We will continue to expand national security in preventing infectious diseases by introducing vaccines that are more effective and safe for children and adolescents."," he said.
This article was translated by Naver AI translator.