Samsung Bioepis Bone Disease Treatment Xbrick Launched in Korea...Boryeong is in charge of sales and marketing
Aug 04, 2025
|
Following the product approval in May, Xbrick signed a partnership agreement with Boryeong for domestic sales of Samsung Bioepis in June, and the salary was applied on the 1st. Samsung Bioepis is in charge of the development, production, and supply of X-brick, while Boryeong is in charge of domestic sales and marketing.
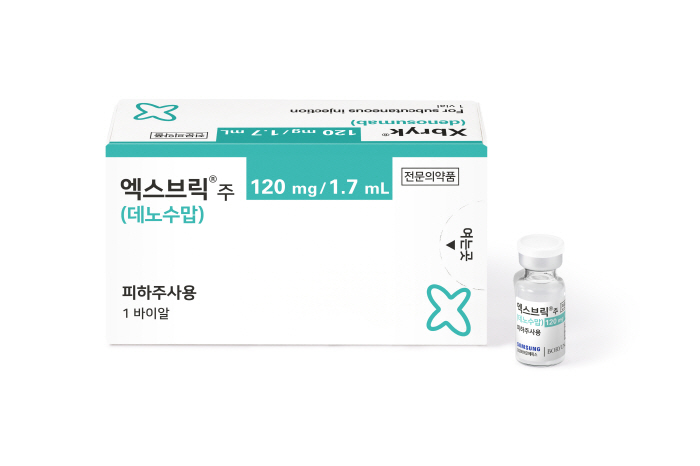
Xbrick, a biosimilar of Xgeva, is used to prevent skeletal symptoms (SRE) and treat giant cell carcinoma in bone metastasis cancer patients. Based on this indication, the global market size of denosumab is about 3.3 trillion won.
Xbrick has demonstrated the same efficacy and safety as the original product in a global phase 3 clinical trial of 456 postmenopausal female patients in five countries, including 41 Koreans, through the SB16 project, and it has been confirmed that the treatment effect is maintained even when the original product is replaced with the patient.
Even after being stored at room temperature (25℃) for up to 60 days, it can be re-stored in the refrigerator, which greatly improves storage convenience compared to the original. In addition, it is the most economical product among Denosumab bone disease treatments with a drug price of 171,084 won per 1.7mL bottle, and is expected to contribute to lowering the burden of medical expenses and increasing access to treatment for patients.
Boryeong plans to focus on expanding prescriptions quickly by adding expertise in the anticancer field to Samsung Bioepis technology and quality. The two companies have already demonstrated synergies through anticancer biosimilar partnerships. Sales of OnBevG (component name Bevacizumab), one of the collaborative items, reached 45.2 billion won last year.
This article was translated by Naver AI translator.