Effect of combination treatment of amivantamab and lasertinib for EGFR mutant lung cancer...a three-year survival rate of 60 percent
Sep 16, 2025
|
A research team led by Professor Cho Byung-chul at Yonsei Cancer Hospital's lung cancer center announced on the 16th that in a multi-national, randomized phase 3 study of EGFR mutant lung cancer patients with no therapeutic power, amivantamab and lasertinib combination therapy reduce the risk of death by 25% compared to osimertinib, a standard treatment.
The findings were published in the international journal 『New England Journal of Medicine』. Professor Cho Byung-chul is the first in Korea to publish NEJM No. 3 in the field of oncology. In addition, it is the first time that clinical results of domestic anticancer drugs are published twice in NEJM.
EGFR-mutated lung cancer accounts for 25-40% of all lung cancers and is the most common type of lung cancer, with 450,000 new cases occurring every year worldwide. Until now, osimertinib, which is used as a first-line treatment, has a response rate of 80% and a progression-free survival period of 16-18 months. After that, most of the patients become resistant.
Professor Cho Byung-chul continued the multi-national, randomized phase 3 this time, as he confirmed the effect of combination therapy in the previous phases 1 and 2. Amivantamab used in combination therapy is a advanced EGFR mutant lung cancer drug, and lasertinib is a mutant lung cancer drug that occurred at exon20 at a specific site of the EGFR gene and has been approved by the Ministry of Food and Drug Safety, respectively. In addition, the combination therapy was approved by the U.S. FDA in August last year due to the effect of increasing progression-free survival period compared to osimertinib previously announced by Professor Cho Byung-chul.
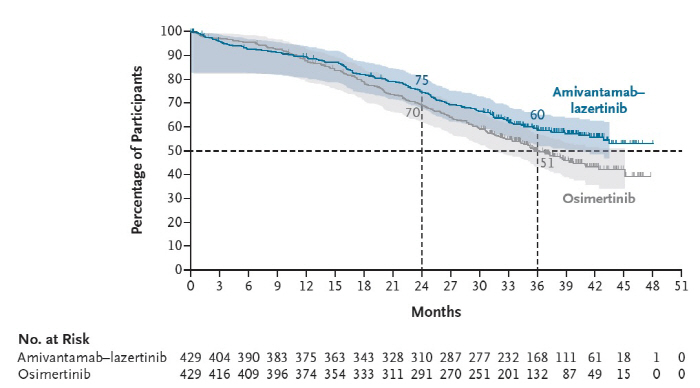
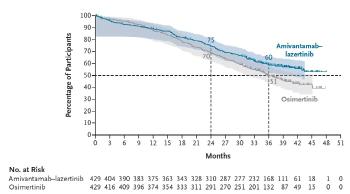
The 3-year survival rate of 429 patients with combination therapy was 60%, an improvement of 9% compared to the osimertinib treatment group. During the observation period of 37.8 months, the median overall survival time in the amivantamab and lasertinib combination groups was beyond the observation period, whereas the osmutinib treatment group recorded 36.7 months.
The combination treatment also showed excellent effects in the patient group with brain metastasis. In addition, the main side effects of the combination therapy were skin rashes and inflammation around the nails, and most of them were inadequate reactions that could be controlled.
Professor Byung-chul Cho "By proving the improvement of progression-free survival period last year and the improvement of overall survival rate this time, we demonstrated the clear therapeutic effect of amivantamab and lasertinib combination therapy in patients with advanced EGFR mutation non-small cell lung cancer."This NEJM announcement suggested the possibility that a first-line chemotherapy-free treatment strategy that uses next-generation targeted treatments beyond chemotherapy will become a new standard treatment."."
|
This article was translated by Naver AI translator.