Seoul National University Hospital Develops an Innovative Treatment Platform Customized for Children with Rare Diseases...14.75 billion won for 4.5 years
Sep 10, 2025
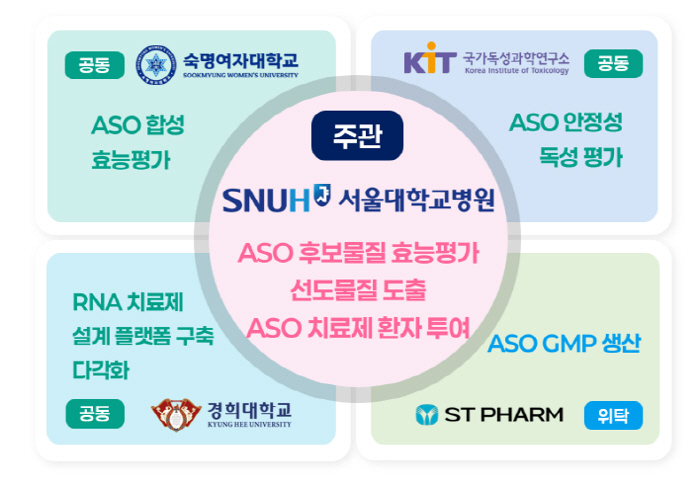
Seoul National University Hospital (Hospital Director Kim Young-tae) has been selected as the lead agency for the 2025 Korean ARPA-H project research project to promote the development of a customized innovative treatment platform for rare diseases in children and N-of-1 clinical trials.
This project is an R&D project supported by the Ministry of Health and Welfare, the Korea Health Industry Promotion Agency, and the K-Health Future Promotion Team, which will provide up to 14.75 billion won over 4.5 years. Seoul National University Hospital will form a consortium with Sookmyung Women's University, the National Institute of Toxic Science, Kyung Hee University, and STpharm, and conduct joint research to present a new paradigm for treating rare diseases in children in Korea.
There are more than 7000 types of rare diseases, and 80% of them occur in childhood. Although the diagnosis rate is increasing based on the development of genomic medicine and institutional support, even if diagnosed, most diseases do not have suitable treatments. In particular, it is difficult to obtain treatment opportunities with the existing new drug development method, which takes an average of more than 10 years for severe life-threatening rare diseases in children. Accordingly, strategies to design drugs according to patient genetic mutations are being attempted around the world for faster drug development.
The Seoul National University Hospital Consortium will use ASO (Antisense Oligonucleotide) technology to design and produce customized treatments for patients with rare diseases. This is a technology that regulates protein expression by combining with mRNAs of specific genes and corrects the underlying cause of the disease, and is the most actively studied in the field of precision medical-based treatment. Furthermore, 'N-of-1 clinical trial', which develops and mediates ASO treatments for only one patient, will be promoted for the first time in Korea.
This study is significant in that it establishes a new rare disease treatment platform through the development of customized gene therapy drugs and clinical trials for patients with severe rare diseases in children. In addition, it is expected to be designed to be extended to patients with other genetic abnormalities to increase treatment accessibility for patients with rare diseases in Korea and provide world-class precision medical-based innovative treatment benefits.
Professor Chae Jong-hee (Department of Clinical Genomics Medicine) at Seoul National University Hospital, the lead researcher, said, "This study will be an important turning point that can give new hope to pediatric patients and their families suffering from rare diseases. We will do our best to apply patient-specific gene therapy to actual patients through this project."."
This project is an R&D project supported by the Ministry of Health and Welfare, the Korea Health Industry Promotion Agency, and the K-Health Future Promotion Team, which will provide up to 14.75 billion won over 4.5 years. Seoul National University Hospital will form a consortium with Sookmyung Women's University, the National Institute of Toxic Science, Kyung Hee University, and STpharm, and conduct joint research to present a new paradigm for treating rare diseases in children in Korea.
There are more than 7000 types of rare diseases, and 80% of them occur in childhood. Although the diagnosis rate is increasing based on the development of genomic medicine and institutional support, even if diagnosed, most diseases do not have suitable treatments. In particular, it is difficult to obtain treatment opportunities with the existing new drug development method, which takes an average of more than 10 years for severe life-threatening rare diseases in children. Accordingly, strategies to design drugs according to patient genetic mutations are being attempted around the world for faster drug development.
The Seoul National University Hospital Consortium will use ASO (Antisense Oligonucleotide) technology to design and produce customized treatments for patients with rare diseases. This is a technology that regulates protein expression by combining with mRNAs of specific genes and corrects the underlying cause of the disease, and is the most actively studied in the field of precision medical-based treatment. Furthermore, 'N-of-1 clinical trial', which develops and mediates ASO treatments for only one patient, will be promoted for the first time in Korea.
This study is significant in that it establishes a new rare disease treatment platform through the development of customized gene therapy drugs and clinical trials for patients with severe rare diseases in children. In addition, it is expected to be designed to be extended to patients with other genetic abnormalities to increase treatment accessibility for patients with rare diseases in Korea and provide world-class precision medical-based innovative treatment benefits.
Professor Chae Jong-hee (Department of Clinical Genomics Medicine) at Seoul National University Hospital, the lead researcher, said, "This study will be an important turning point that can give new hope to pediatric patients and their families suffering from rare diseases. We will do our best to apply patient-specific gene therapy to actual patients through this project."."
|
This article was translated by Naver AI translator.