Domestic Folio Vaccine Production Facility Gets WHO Airtight Certification for the Third Time in the World...For the first time in Asia
|
LG Chem's polio vaccine production facility established an injection-type polio vaccine production line in 2018 and has been supplying UNICEF vaccines since 2021 after WHO's prior eligibility assessment (PQ) approval in 2020. As of 2024, 40.86 million doses, with a 35% share of the injectable polio vaccine.
According to the Korea Centers for Disease Control and Prevention, the WHO's acquisition of closed certification for essential polio facilities in Korea is significant in that it has been internationally recognized for its national biosafety management capabilities as well as the safety of polio vaccine production facilities. In addition to handling poliovirus, it has safety and risk management capabilities that can be used as a development and production facility for new infectious disease vaccines that are at high risk in the future.
The WHO has established the Global Plan for Elimination of Polio (GPEI) since 1988, requires extensive vaccination by country to eradicate polio (pneumonia), and requires that polio essential facilities be certified by 2026 by the World Health Organization.
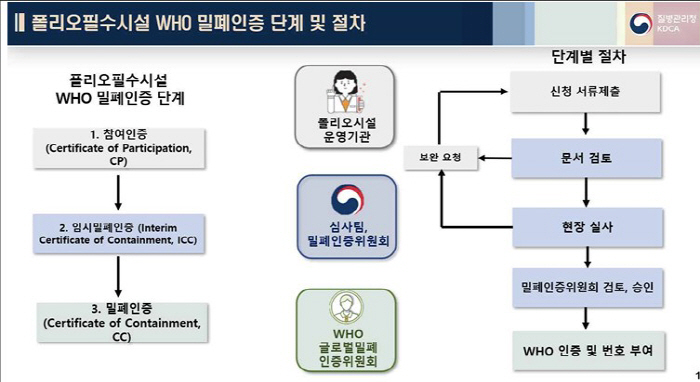
WHO closed certification is a procedure for evaluating closed facility standards and hazard management systems for safe handling of poliovirus. It is based on WHO guidelines 'Global Action Plan 4th Edition, GAPIV'. If all 14 detailed standards are met, including biological hazard management systems, education and training, security, physical sealing, and emergency response plans, it is possible to obtain closed certification for essential polio facilities.
Since 2018, the Korea Centers for Disease Control and Prevention has formed the National Enclosure Certification Committee to support risk management education and conduct on-site inspections of essential folio facilities, and continued technical support and cooperation between the WHO for enclosure certification of essential folio facilities, including participation in WHO response training.
Director of the Korea Centers for Disease Control and Prevention Lim Seung-kwan said, "This acquisition of WHO polio airtight certification served as an opportunity for Korea's advanced biosafety national management system and risk management capabilities of domestic polio vaccine-producing companies to be recognized internationally." The Korea Centers for Disease Control and Prevention will conduct additional national surveys on polio-infectious substances implemented under the WHO's Global Polio Eradication Plan and actively participate in international implementation efforts to eradicate polio."," he said.
This article was translated by Naver AI translator.