GLP1 obesity treatments such as Hugo Bee have been introduced, but...Prescribing psychotropic appetite suppression only slightly decreased
|
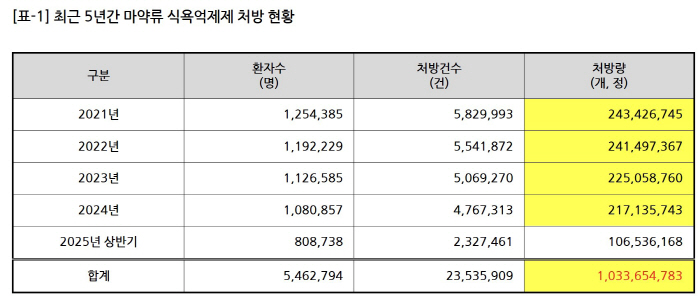
According to the data submitted by Kim Sun-min (Cho Kuk Innovation Party), a member of the National Assembly's Health and Welfare Committee, from the Ministry of Food and Drug Safety, the cumulative prescription for drug appetite suppressants in the first half of 2021 to 2025 was 1.03365 million. Annual prescriptions decreased slightly from 243.42 million tablets in 2021 to 217.13 million tablets in 2024, but more than 200 million tablets are still prescribed every year. Over the past five years, the cumulative prescription for drug appetite suppressants has exceeded 1 billion.
By major ingredients, as of last year, 700,000 people were prescribed ▲ pentamine with side effects such as insomnia and anxiety, 500,000 people were prescribed ▲ pentimetrazine, and more than 70,000 people were prescribed ▲ amphiphramone.
In particular, 969,341 (89.7%) female patients out of 1.08 million patients prescribed these appetite suppressants, nearly nine times more than men (111,516). In addition, 550,000 appetizers were prescribed to 5,899 teenagers under the age of 10. The number of foreign prescription patients also steadily increased from 34,063 in 2021 to 43,804 in 2024.
This is pointed out as the background of the standard for prescribing appetite suppressants.
In the UK, France, Japan, and the United States, prescriptions are only allowed for body mass index (BMI), which is the value of body weight divided by the square of height, and psychotropic appetite suppressants themselves are prohibited in the UK and France. On the other hand, in Korea, a BMI of 23 or higher is recognized as a pre-obesity stage, and in fact, a wide range of prescriptions are possible.
Reports of major side effects such as insomnia, heart palpitations, and dizziness for drug appetite inhibitors have also continued to increase recently. In particular, in 2024, there were 455 cases, including ▲ 68 cases ▲ 50 cases of tardiness, the highest in the past five years.
Despite the annual reports of side effects, systematic monitoring, management, and supervision of the status of abuse are still insufficient.
According to actual data from the Ministry of Food and Drug Safety, only 11 (0.3%) of 3,636 doctors who received a 'pre-notification' warning for off-the-shelf drug appetite suppressor misuse measures in the past four years were requested for administrative disposition.
Representative Kim Sun-min said "More than 200 million appetite suppressants are used annually amid the blurring of social appearance pressure and medical judgment""The misuse of youth and women, loose BMI standards, and insufficient follow-up management systems should no longer be neglected" he said. It is urgent to conduct an in-depth investigation into the current status of prescribing appetite suppressants for women and adolescents and to strengthen standards. The standards for prescribing appetite suppressants for drugs should be reorganized to the level of advanced countries for the safety and mental health of the people, and the management and supervision system should be urgently strengthened to prevent soft punishment," he stressed.
This article was translated by Naver AI translator.