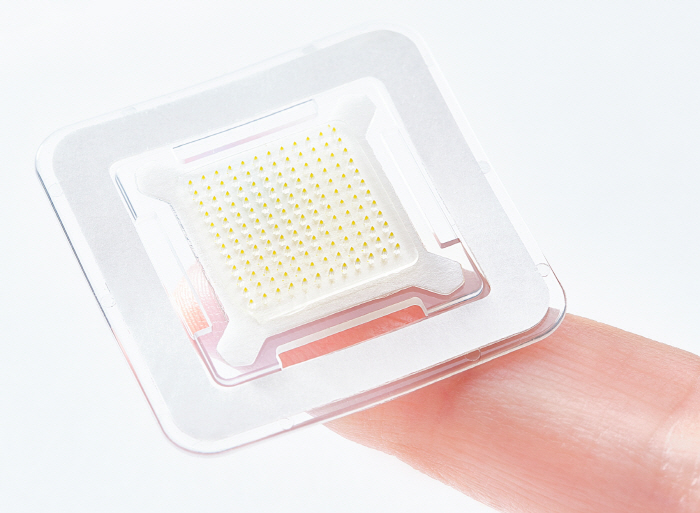
Semaglutide component microneedle patch attached once a week to Daewoong Pharmaceutical and Daewoong Therapeutics enters phase 1 clinical trial
|
It is a clinical trial that evaluates the safety and pharmacokinetic characteristics of microneedle patches in healthy adults and confirms the relative bioavailability compared to Novonodisk's obesity and diabetes treatments 'Ozempic' and 'Wigobi'.
Daewoong's microneedle patch is a patchy formulation that dissolves fine needles composed of semaglutide when attached to the skin and delivers the drug directly to the skin dermis once a week. Daewoong Therapeutics' proprietary drug delivery platform 'Clopharm', which maximizes drug uniformity and stability through differentiated technologies such as pressure drying and completely close packaging, and enables precise administration without concerns of contamination.
Clopharm has a high relative bioavailability of more than 80% compared to subcutaneous injections, which is significantly higher than the 30% level of existing microneedle patches released so far and about 160 times higher than oral drugs. This is expected to reduce the burden of taking oral drugs, increase compliance with treatment compared to injection, and reduce the time required for injection administration and monitoring, allowing medical staff to focus more on patient management, resulting in increased treatment efficiency.
Kang Bok-ki, CEO of Daewoong Therapeutics, said "This clinical entry is an important first step to demonstrate the global competitiveness of our microneedle platform" and "We will change the paradigm of obesity treatment through new formulations with safety and efficiency and show the possibility that domestic technology can work in the global market"
Park Sung-soo, CEO of Daewoong Pharmaceutical, said, `Daewoong Pharmaceutical wants to go beyond technology-based new drug development to introduce innovative formulations that are recognized in the global market.''"The once-weekly patch formulation will be the next-generation option to increase patient compliance and improve efficiency in the medical field.'
This article was translated by Naver AI translator.