Starting today, pneumococcus 20 introduces a national vaccination against a protein-binding vaccine
From today, the pneumococcal 20 protein-binding vaccine (hereinafter 'PCV20') will be newly introduced into the National Vaccination Project (NIP).
Pneumococcus pneumoniae is a major bacterial pathogen that causes various diseases such as otitis media, pneumonia, and meningitis in infants and toddlers, and vaccination is very important because it can cause Invasive Pnemoccal Disease (IPD), which can be life-threatening, especially in children with weak immune systems.
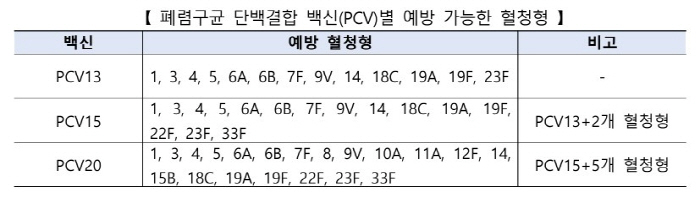
In the existing National Vaccination Project (NIP), 13-valent protein-binding vaccines (hereinafter referred to as 'PCV13') and 15-valent protein-binding vaccines (hereinafter referred to as 'PCV15') were supported for childhood pneumocococcal vaccination, and the PCV20 vaccine introduced this time increased the number of preventable serotypes in the previously inoculated PCV15 by five types (8, 10A, 11A, 12F, and 15B), increasing the effectiveness.
PCV20, which was approved by the Ministry of Food and Drug Safety in October last year, was decided to be introduced by a specialized vaccination committee after comprehensively reviewing the safety, immunogenicity, and cost-effectiveness of the vaccine.
Infants under 59 months of age and high-risk groups for pneumococcus under 18 years of age (immunocompromised, chronically ill, etc.) are eligible for inoculation. The inoculation schedule for healthy children is the same as before, with a total of three inoculations at 2, 4, and 6 months of age and one additional inoculation every 12 to 15 months, and children who have already started inoculation with PCV13 can be cross-vaccinated with PCV20. However, if inoculation with PCV15 is started, the same vaccination is recommended.
From October 1, it can be inoculated at entrusted medical institutions nationwide, and abnormal reactions are required for 20-30 minutes after inoculation.
Pneumococcus pneumoniae is a major bacterial pathogen that causes various diseases such as otitis media, pneumonia, and meningitis in infants and toddlers, and vaccination is very important because it can cause Invasive Pnemoccal Disease (IPD), which can be life-threatening, especially in children with weak immune systems.
|
PCV20, which was approved by the Ministry of Food and Drug Safety in October last year, was decided to be introduced by a specialized vaccination committee after comprehensively reviewing the safety, immunogenicity, and cost-effectiveness of the vaccine.
Infants under 59 months of age and high-risk groups for pneumococcus under 18 years of age (immunocompromised, chronically ill, etc.) are eligible for inoculation. The inoculation schedule for healthy children is the same as before, with a total of three inoculations at 2, 4, and 6 months of age and one additional inoculation every 12 to 15 months, and children who have already started inoculation with PCV13 can be cross-vaccinated with PCV20. However, if inoculation with PCV15 is started, the same vaccination is recommended.
From October 1, it can be inoculated at entrusted medical institutions nationwide, and abnormal reactions are required for 20-30 minutes after inoculation.
This article was translated by Naver AI translator.