Dong-A ST/Metavia Announces Poster of Global Phase 1 Clinical and Preclinical Data for Obesity Treatment DA1726 at the Society of Obesity
Nov 05, 2025
|
Metabia is a Nasdaq-listed company located in Boston, USA, and is a global R&D forward base of Dong-A Socio Group in charge of global development and commercialization of MASH (Metabolic Dysfunction-associated steatohepatitis) treatments DA-1241 and DA-1726.
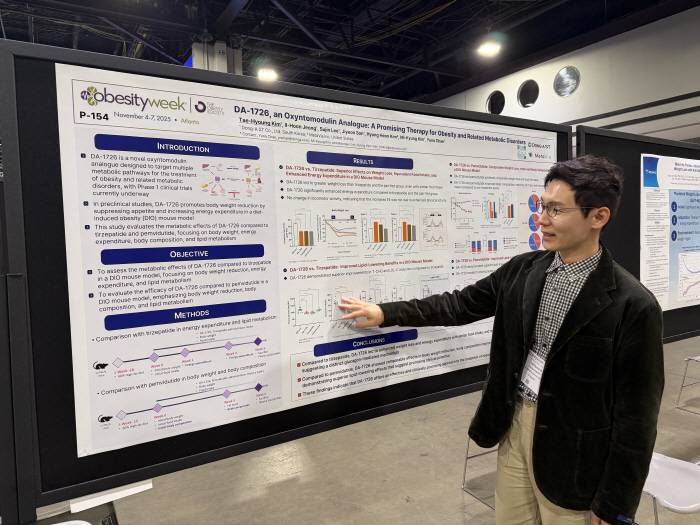
DA-1726 is a new drug candidate being developed as a treatment for obesity in the Oxyntomodulin analogue (oxyntomodulin analogue). It acts simultaneously on GLP-1 and Glucagon receptors, promoting appetite suppression, insulin secretion, and increasing basal metabolism in the periphery, ultimately leading to weight loss and glycemic control.
Phase 1 clinical trials were randomized, double-blinded, and placebo-controlled in nine obese adults to identify safety, tolerability, pharmacokinetics, and pharmacokinetics in single and multiple doses of DA-1726. DA-1726 32 mg was administered subcutaneously once a week for 4 weeks without dose titration.
As a result of the study, the DA-1726 administration group lost up to 6.3% (6.8 kg) and an average of 4.3% (4.0 kg) in 26 days of administration. The waist circumference decreased by up to 3.9 inches (10 cm), and the effect lasted for two weeks after the end of the medication. In addition, the dose-linear pharmacokinetic characteristics and an average half-life of 80 hours were checked to confirm the possibility of once-week administration.
The preclinical study newly released at this conference was conducted on a high-fat diet-induced obesity (DIO) mouse model. DA-1726 promoted weight loss through appetite suppression and increased energy consumption. Despite similar food intake compared to Tirzepatide, it showed excellent weight loss because it significantly increased basal metabolism compared to Tirzepatide. This increase in energy consumption was significantly high without any change in exercise activity. It also further reduced total cholesterol (T-CHO) and LDL-C and demonstrated metabolic mechanisms following glucagon receptor action.
Compared to the same substance, pembidutide (pembidutide), the weight loss effect was similar, and body composition improvement was similarly confirmed through fat reduction and relative fat preservation. It also significantly reduced total cholesterol, LDL-C, and triglycerides, and showed excellent lipid improvement effect.
MetaVia has been conducting an additional Phase 1 clinical trial to explore maximum tolerable dose (MTD) at a 48 mg dose for a total of eight weeks since July. The data is scheduled to be released later this year to demonstrate better weight loss effects, safety, and tolerability.
CEO of Metavia, Kim Hyung-heon, "In Phase 1 clinical trials, we confirmed excellent safety, initial weight loss, waist circumference reduction, and cardiovascular safety, and demonstrated the possibility of once-a-week obesity treatment through pharmacokinetic characteristics and 80-hour half-life."DA-1726 is strengthening its competitiveness as a differentiated obesity treatment, and we will further demonstrate its competitiveness through additional Phase 1 clinical trials to explore the current maximum drug dose.'
This article was translated by Naver AI translator.