Rare genetic disease Lever's optic nerve atrophy succeeded in gene correction treatment for the first time in the world
|
Leber Heritable Optic Neurophy (LHON) is a maternal inherited mitochondrial disease that causes rapid central field of vision loss and blindness due to degeneration of optic cells. It occurs mainly in young men in their 10s and 30s, and is known as a major genetic optic nerve disease that causes more than 30,000 to 50,000 people worldwide to lose sight.
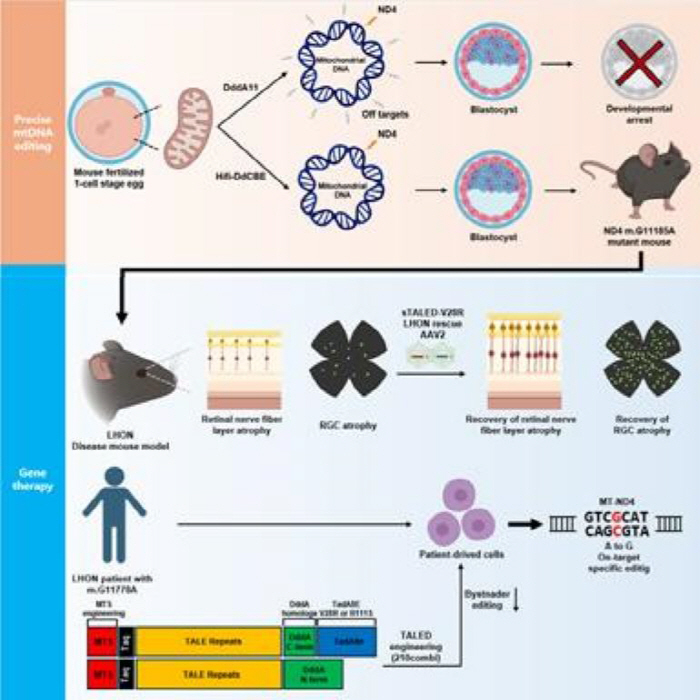
The cause of LHON is point mutations in complex I genes such as MT-ND4, ND1, and ND6 within mitochondrial DNA (mtDNA). Among them, m of MT-ND4.The G11778A variant accounts for approximately 70% of all patients. However, gene therapy was not possible because the guide RNA of typical CRISPR gene scissors could not enter the mitochondrial interior. Idebenone, the only treatment currently approved, also temporarily aids mitochondrial function, but has limited underlying therapeutic effects. For this reason, the establishment of accurate disease models and the development of fundamental correction techniques were considered urgent tasks worldwide.
The research team precisely implemented MT-ND4 G11778A mutation (m.G11185A) corresponding to human LHON mutation in mice using High-Fidelity DdCBE (Hifi-DdCBE), a mitochondrial base correction technology. This model showed the same pathological characteristics as actual LHON patients, such as decreased retinal optic nerve layer thickness, decreased optic nerve cell count, and decreased visual function.
Since then, the research team has applied a treatment to correct the mutated mtDNA to a normal base by delivering it to AAV (adenoside virus) using TALE-linked deninase (TALED-V28R), which was reported in the 2024 Cell paper. AAV-TALED V28R injected via intraclastic injection corrected genetic variation, a pathogenesis of optic nerve cells. Retinal thickness and optic nerve cell count were restored to normal levels, and visual function (OKN·ERG test) was also significantly improved. In addition, as a result of applying TALED V28R to LHON patient-derived cells, ATP production and mitochondrial complex I activity were restored, providing the basis for clinical applicability.
Co-author Kim Sang-hoon, a Ph.D. student at Korea University Medical School, said, `There will be new possibilities and hopes for patients suffering from the absence of therapeutic agents,' adding, `We will further develop mitochondrial gene correction technology in the future so that it can be used in actual clinical settings.'
"This is the first study in the world to demonstrate the effectiveness of gene correction treatment in a living model of mitochondrial disease," said Lee Hyun-ji, co-director of the study. "We hope that gene correction treatment for various mitochondrial genetic diseases, including Lever's optic atrophy, will jump to clinical application and be commercialized as an actual treatment."," he said.
On the other hand, the study was published in the world-renowned international journal Nature Communications' under the title In Vivo Mitochondrial Base Editing Genotype and Visual Function in a Mouse Model of LHON' by correcting mitochondrial bases in vivo.
|
N2MT07||text parameter is needed (text 파라미터가 필요합니다.)
This article was translated by Naver AI translator.