Last year, rare drug licenses continued to increase...Biosimilar Permission Highest Ever
Apr 29, 2025
Amid the increase in rare drug licenses last year, equivalent biopharmaceutical (biosimilar) licenses recorded the highest ever.
According to the 2024 approval report published by the Ministry of Food and Drug Safety on the 29th, a total of 1,197 drugs were approved and reported last year.
As the main approval trend, ▲Rare drug license continues to increase ▲'Circulating drug' is the largest proportion ▲ is the highest approval in the history of equal biopharmaceuticals (biosimilars).
A total of 26 ingredients and 39 items were approved for rare drugs, up two from last year, and among them, 16 items are anti-malignant tumors, including acute myeloid leukemia treatment.
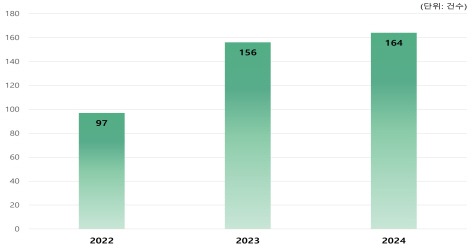
In 2024, equivalent biopharmaceuticals were approved for a total of 18 items (10 ingredients), the largest number since the first product approval in 2012. Of these, 13 items with seven components, or more than half, are domestic development items, and 52 items (24 components) out of a total of 72 items (35 components) licensed by 2024 are domestic development items, accounting for about 72%. It is expected that domestic companies will continue to actively develop equivalent biopharmaceuticals as there are many items from original developers about to expire their patents.
In addition, last year, circulatory drugs containing ginkgo leaf dry X were approved the most with a total of 161 items, followed by fever, pain, and anti-inflammatory drugs (146 items), diabetic drugs (127 items), other vitamin drugs (99 items), and anti-malignant tumor drugs (39 items).
The number of licensed and reported items such as generic drugs (replicated drugs) totaled 845, a slight increase compared to 2023. This seems to be the effect of limiting the number of times the same clinical trial data can be used for other items to three times from July 2021, and the system has settled stably in view of similar levels in the last three years.
A total of 659 items of non-drug drugs have been approved and reported. Major permit trends in 2024 include ▲ continuous development of new items such as safety and effectiveness screening ▲ maintaining the dominant trend of licensing and reporting domestic manufacturing items.
Last year, a total of 10 items and 36 items were approved for safety and effectiveness, confirming that the development of non-drug products related to daily life, such as Band-Aids with new materials and contact lens management products, continues.
In addition, the number of permits and reports of domestic manufacturing items last year was 567, accounting for 86% of the total number of permits and reports (659), and domestic manufacturing items are considered to be highly competitive in quality as they remain dominant.
By non-drug product group, sanitary pads accounted for the highest proportion with 41.1 percent (271 items), followed by toothpaste and bandages with 19.3 percent and 10.5 percent, respectively.
A total of 7,116 medical devices have been approved, certified, and reported. The main licensing trends in 2024 include ▲ Increasing number of independent digital medical device software (SaMD) items ▲ Increasing tissue restoration and skin-related medical device items ▲ Silver medical devices include high-frequency licensing items every year due to the aging population.
First of all, as the performance of artificial intelligence (AI)-based SaMD improved, Korea's first licensed products stood out in various medical fields last year. In particular, 33 (80.5%) of the total 41 items of innovative medical devices licensed by 2024 were counted as SaMD products.
In addition, as interest in domestic and foreign skin care and plastic surgery increases, skin-related medical devices such as tissue repair and wrinkle improvement are steadily being approved.
In addition, as the prevalence of tooth loss and related diseases increases due to the aging population, the top items in the frequency of licensing, certification, and reporting in 2024 included 'Dental Implant Upper Structures'(135 cases, 3rd place) and 'Dental Implant Treatment Instruments'(98 cases, 6th place).
According to the 2024 approval report published by the Ministry of Food and Drug Safety on the 29th, a total of 1,197 drugs were approved and reported last year.
As the main approval trend, ▲Rare drug license continues to increase ▲'Circulating drug' is the largest proportion ▲ is the highest approval in the history of equal biopharmaceuticals (biosimilars).
A total of 26 ingredients and 39 items were approved for rare drugs, up two from last year, and among them, 16 items are anti-malignant tumors, including acute myeloid leukemia treatment.
In 2024, equivalent biopharmaceuticals were approved for a total of 18 items (10 ingredients), the largest number since the first product approval in 2012. Of these, 13 items with seven components, or more than half, are domestic development items, and 52 items (24 components) out of a total of 72 items (35 components) licensed by 2024 are domestic development items, accounting for about 72%. It is expected that domestic companies will continue to actively develop equivalent biopharmaceuticals as there are many items from original developers about to expire their patents.
In addition, last year, circulatory drugs containing ginkgo leaf dry X were approved the most with a total of 161 items, followed by fever, pain, and anti-inflammatory drugs (146 items), diabetic drugs (127 items), other vitamin drugs (99 items), and anti-malignant tumor drugs (39 items).
The number of licensed and reported items such as generic drugs (replicated drugs) totaled 845, a slight increase compared to 2023. This seems to be the effect of limiting the number of times the same clinical trial data can be used for other items to three times from July 2021, and the system has settled stably in view of similar levels in the last three years.
A total of 659 items of non-drug drugs have been approved and reported. Major permit trends in 2024 include ▲ continuous development of new items such as safety and effectiveness screening ▲ maintaining the dominant trend of licensing and reporting domestic manufacturing items.
Last year, a total of 10 items and 36 items were approved for safety and effectiveness, confirming that the development of non-drug products related to daily life, such as Band-Aids with new materials and contact lens management products, continues.
In addition, the number of permits and reports of domestic manufacturing items last year was 567, accounting for 86% of the total number of permits and reports (659), and domestic manufacturing items are considered to be highly competitive in quality as they remain dominant.
By non-drug product group, sanitary pads accounted for the highest proportion with 41.1 percent (271 items), followed by toothpaste and bandages with 19.3 percent and 10.5 percent, respectively.
|
First of all, as the performance of artificial intelligence (AI)-based SaMD improved, Korea's first licensed products stood out in various medical fields last year. In particular, 33 (80.5%) of the total 41 items of innovative medical devices licensed by 2024 were counted as SaMD products.
In addition, as interest in domestic and foreign skin care and plastic surgery increases, skin-related medical devices such as tissue repair and wrinkle improvement are steadily being approved.
In addition, as the prevalence of tooth loss and related diseases increases due to the aging population, the top items in the frequency of licensing, certification, and reporting in 2024 included 'Dental Implant Upper Structures'(135 cases, 3rd place) and 'Dental Implant Treatment Instruments'(98 cases, 6th place).
This article was translated by Naver AI translator.