The world's first investigation into why aging cells spread throughout the body
Apr 21, 2025
For the first time in the world, domestic researchers have discovered why aging cells spread throughout the body. In addition, it is attracting attention by presenting a new control strategy that can prevent this process.
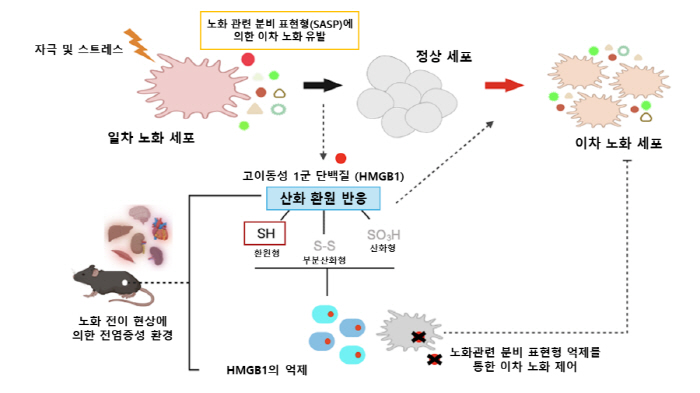
Professor Jeon Ok-hee's research team at Korea University's School of Convergence Medicine has discovered that the protein 'HMGB1 (High Mobility Group Box 1)' plays a key role in spreading cell aging throughout the body.
One of the typical characteristics of senescent cells is to make the surrounding cells age together. These cells are characterized by aging even other normal cells by sending out inflammatory substances and 'aging-inducing signals' around them. This is called 'aging-associated secretion phenotype (SASP)'. As these senescent cells accumulate in various tissues, the function of the entire body decreases and the ability to recover gradually decreases.
Through experiments, the research team confirmed that HMGB1 secreted from senescent cells spreads throughout the body through blood and induces aging of normal cells and tissues. In particular, it has been found to inhibit tissue regeneration and cause functional degradation in muscle tissue. In addition, the team noted that the form 'ReHMGB1 (reduced HMGB1)' is responsible for spreading aging. This protein is not just a common substance from old cells, it may be a key factor in spreading aging.
To reduce the activity of HMGB1, the team administered antibodies that block it to mice. As a result, systemic inflammation was reduced and the regeneration and function of damaged muscles were greatly improved. In addition, it was also confirmed that the aging-inducing effect was reduced when HMGB1 blocked the RAGE receptor, a pathway for signaling to cells.
In addition, the research team has announced that a non-cell autonomous mechanism called 'transmission of aging cell-derived substances in aging blood can cause aging and related diseases. On the other hand, this study is significant in that it does not just identify aging cells, but also identifies the process of aging spreading throughout the body through blood. In particular, it is evaluated as a study that revealed the principle of action of HMGB1, a protein that spreads aging, and showed the possibility of tissue function recovery by controlling it.
Professor Jeon Ok-hee said, "This study reveals a molecular mechanism that can explain the phenomenon of 'aging metastasis' in which aging spreads throughout the body through blood, not just to specific cells or tissues. It is significant in that it has presented a new strategy to treat aging-related diseases as it has confirmed the possibility of reviving tissue function if this process is blocked."
The study was conducted by the Ministry of Science and ICT's Myocaine Convergence Research Center (MRC) and mid-sized research support projects, and was conducted in collaboration with world-renowned researchers in aging, including UC Berkeley Professor Irina Conboy and Turfts University Professor Christopher Wiley.
On the other hand, the research results were recently published in Metabolism-Clinical and Experimental (IF: 10.9, Top 4.6%), a prestigious journal in the field of endocrine metabolism under the title Propagation of senescent phenotypes by extracellular HMGB1 depends on the redox state.
Professor Jeon Ok-hee's research team at Korea University's School of Convergence Medicine has discovered that the protein 'HMGB1 (High Mobility Group Box 1)' plays a key role in spreading cell aging throughout the body.
One of the typical characteristics of senescent cells is to make the surrounding cells age together. These cells are characterized by aging even other normal cells by sending out inflammatory substances and 'aging-inducing signals' around them. This is called 'aging-associated secretion phenotype (SASP)'. As these senescent cells accumulate in various tissues, the function of the entire body decreases and the ability to recover gradually decreases.
Through experiments, the research team confirmed that HMGB1 secreted from senescent cells spreads throughout the body through blood and induces aging of normal cells and tissues. In particular, it has been found to inhibit tissue regeneration and cause functional degradation in muscle tissue. In addition, the team noted that the form 'ReHMGB1 (reduced HMGB1)' is responsible for spreading aging. This protein is not just a common substance from old cells, it may be a key factor in spreading aging.
To reduce the activity of HMGB1, the team administered antibodies that block it to mice. As a result, systemic inflammation was reduced and the regeneration and function of damaged muscles were greatly improved. In addition, it was also confirmed that the aging-inducing effect was reduced when HMGB1 blocked the RAGE receptor, a pathway for signaling to cells.
In addition, the research team has announced that a non-cell autonomous mechanism called 'transmission of aging cell-derived substances in aging blood can cause aging and related diseases. On the other hand, this study is significant in that it does not just identify aging cells, but also identifies the process of aging spreading throughout the body through blood. In particular, it is evaluated as a study that revealed the principle of action of HMGB1, a protein that spreads aging, and showed the possibility of tissue function recovery by controlling it.
Professor Jeon Ok-hee said, "This study reveals a molecular mechanism that can explain the phenomenon of 'aging metastasis' in which aging spreads throughout the body through blood, not just to specific cells or tissues. It is significant in that it has presented a new strategy to treat aging-related diseases as it has confirmed the possibility of reviving tissue function if this process is blocked."
The study was conducted by the Ministry of Science and ICT's Myocaine Convergence Research Center (MRC) and mid-sized research support projects, and was conducted in collaboration with world-renowned researchers in aging, including UC Berkeley Professor Irina Conboy and Turfts University Professor Christopher Wiley.
On the other hand, the research results were recently published in Metabolism-Clinical and Experimental (IF: 10.9, Top 4.6%), a prestigious journal in the field of endocrine metabolism under the title Propagation of senescent phenotypes by extracellular HMGB1 depends on the redox state.
|
This article was translated by Naver AI translator.