Phase 1 clinical trial of gene-targeted therapy for lung cancer, colon cancer, and pancreatic cancer...Twice the effectiveness compared to existing treatments
May 12, 2025
|
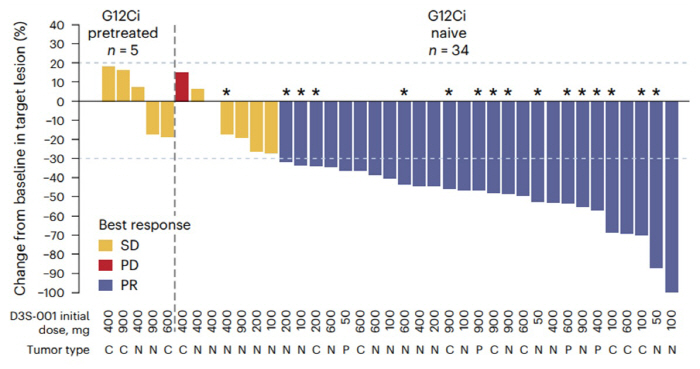
A research team led by Professor Cho Byung-chul and Lim Sun-min at Yonsei Cancer Hospital's lung cancer center announced on the 12th that 73.5% of solid cancer patients such as non-small cell lung cancer, colon cancer, and pancreatic cancer showed in the first phase of the next-generation KRAS G12C targeted treatment.
The study was published in Nature Medicine (IF 58.7), a world-renowned medical journal.
KRAS gene is a gene that regulates cell growth and division. KRAS G12C mutation is the most common mutation that can be found in 25% of non-small cell lung cancers. It is also a cause of other solid cancers such as colon cancer and pancreatic cancer.
Sotorasib is the only KRAS G12C targeted treatment approved by the Ministry of Food and Drug Safety. According to clinical data, the objective response rate is 37.1%, the median progression-free survival period is 6.8 months, and the overall survival period is only 12.5 months. This is the background in which next-generation targeted treatments are needed.
The research team confirmed the results of phase 1 clinical trials of KRAS G12C next-generation targeted therapy.
Patients with colon cancer (9 patients) and pancreatic cancer (4 patients) along with non-small cell lung cancer (21 patients) participated in the phase 1 clinical trial. The objective response rate, which means tumor reduction of more than 30%, recorded 73.5%. Lung cancer, colon cancer, and pancreatic cancer reached 66.7%, 88.9%, and 75%, respectively.
Patients who failed treatment with existing drugs were also able to confirm positive effects. Of the 20 patients with non-small cell lung cancer who had no improvement with existing drugs, 60% showed tumor reduction and the objective response rate reached 30%.
Patients with colorectal cancer who not only had previous treatment resistance but also metastasized to the liver responded well. 78.4% of patients with an objective response maintained a treatment response over 6 months. Notably, 68.6% of all clinical patients did not show disease progression for more than 6 months.
Professor Cho Byung-chul said, "Based on the encouraging phase 1 study, we are conducting a multi-national clinical study to confirm the effectiveness of combination therapy as well as alone. As many patients quickly become resistant to existing targeted treatments, it is important to improve the grades of next-generation targeted treatments such as this drug."
|
This article was translated by Naver AI translator.