Medical device information search service Medical device safety bookstore AI and short-form increase accessibility
Sep 15, 2025
|
The medical device safety book (check) website is a medical device information providing portal that means checking medical devices properly with the public and using them safely, and 4.6 million people have visited it annually since January last year.
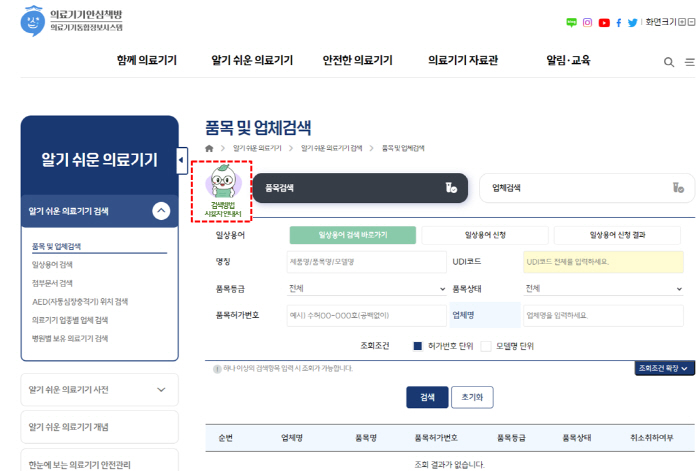
The Medical Device Safe Bookstore's 'item and company search service' provided various search items in consideration of the scope of information use by users, but the first-time users had difficulty due to the complex search method and guidance.
Accordingly, this improvement intuitively explained the complex search process by easily rewriting the search guide in everyday conversation and providing it as a short video (short video). In particular, on the video shortform, the medical device promotion character 'Booki' learns real speech with a generative AI voice cloning technique and explains the search method with a natural voice. The short form using 'Booki' introduces not only search methods, but also digital medical products and medical device safety usage information, and will be used as an effective promotional method in line with changes in trends.
The Ministry of Food and Drug Safety said it will continue to improve medical device safety bookstores in three stages by the end of this year, including this improvement.
In the second stage, which will be implemented in November, it will be possible to check the separate permit information DB search and website content search on one screen, and provide electronic braille data for the visually impaired and the ability to view attached files immediately without downloading them.
In the third phase, which will be completed in December, the company plans to disclose the digital medical device product license information DB under the Digital Medical Products Act (enforced in 2025), search for the latest digital medical product guidelines based on RAG (the Generative AI generates an answer based on the search information), and build a medical device safety bookstore for foreigners in English.
The Ministry of Food and Drug Safety said it plans to make it easy and quick for anyone to check medical device information through step-by-step improvements and do its best to provide services in line with the latest trend changes.
This article was translated by Naver AI translator.