Developing a quick test method to determine whether drugs are sterile in just one day...Existing method takes 14 days
Oct 14, 2025
|
Professor Kwon Sung-hoon of the Department of Electrical and Information Engineering at Seoul National University of Technology (SNU), Professor Lee Eun-joo of the Department of Medical Life Sciences at Seoul National University Hospital, and Professor Kim Tae-hyun of the Graduate School of Convergence at KU-KIST at Korea University have achieved this achievementIt was recently published in Nature Biomedical Engineering'.
The aseptic test method according to Korean Pharmaceutical is a test method that checks whether a product that should be sterile, such as pharmaceuticals or medical devices, is actually sterile and generally takes 14 days. The same confirmation test method is applied to biopharmaceuticals such as cell and gene therapy drugs that have recently emerged in the pharmaceutical market. Biopharmaceuticals are products of modern biotechnology, including CAR-T cell therapy that cured patients with incurable blood cancer, COVID-19 mRNA vaccines that changed the course of the pandemic, and monoclonal antibodies that have become a key weapon in cancer immunotherapy.
These drugs have paved a new way for the treatment of incurable diseases, such as curing cancer, which was considered an incurable area, and providing new options for patients who did not have the opportunity to treat it. As its importance is highlighted as the core of future medical care, the global biopharmaceutical market is estimated to be worth about 400 billion dollars (about 550 trillion won) as of 2024, and is expected to continue to grow by more than 15% every year.
However, in contrast to rapid market growth and technological advances, the quality control standards for biopharmaceuticals remain in compliance with the standards of synthetic drugs in the past. This criterion creates a big problem because it does not reflect the characteristics of biopharmaceuticals such as the latest cell and gene therapy drugs that have an expiration date of only a few days.
While waiting for the results of the biopharmaceutical aseptic test, the effectiveness of the drug disappears and the patient may miss the opportunity to treat it. Eventually, there are cases in which the drug cannot but be administered to the patient without the completion of aseptic proof of the drug. This was an inevitable limitation between patient safety and treatment effectiveness.
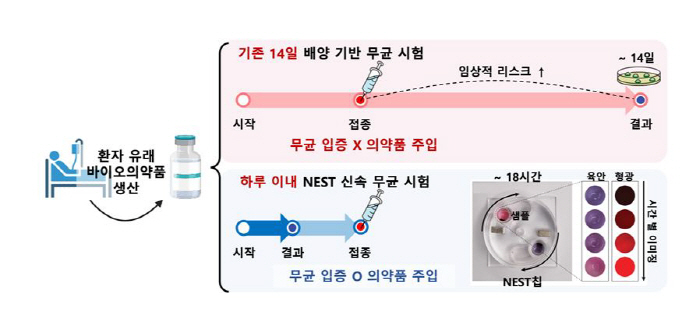
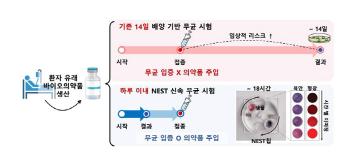
In response, the joint researchers developed NEST, a rapid aseptic test technology that shortened the 14-day period required for the existing test to less than a day. It opened up the possibility of safely administering biopharmaceuticals proven sterile to patients on time.
The researchers took the idea from our body's innate immune response (a defense system that distinguishes invasive bacteria) and quickly concentrated trace amounts of pathogens in pharmaceuticals using special peptide-coated magnetic nanoparticles that selectively bind to various pathogens. Subsequently, it has developed a dedicated imaging chip and automation equipment that can detect a wide range of microbial metabolic signals in real time, securing speed and high reliability at the same time. In particular, it was confirmed that, within a minimum of 5 h to a maximum of 18 h, a trace amount of bacteria (one mycelium per ml) could be detected in 14 species.
In addition, the joint researchers conducted verification using samples for actual administration, such as clinical-grade stem cells and CAR-T cell therapy drugs that cost hundreds of millions of won per dose, and confirmed that NEST works stably in real patient samples beyond the laboratory stage.
Professor Lee Eun-joo of the Department of Life Sciences at Seoul National University Hospital said "In the case of biopharmaceuticals that are immediately administered to patients immediately after manufacturing in the field of advanced regenerative medicine, treatment was inevitable without completion of the 14-day aseptic verification of the completion."If the completion can be confirmed aseptic on the same day and administered to patients in the future, changes will follow in the relevant regulatory system and patient safety will also be improved dramatically."
Quanta Matrix Co., Ltd., a comprehensive microorganism diagnosis company represented by Professor Kwon Sung-hoon, and Seoul National University Hospital signed a memorandum of understanding (MOU) for rapid aseptic verification research and commercialization in August for the field application of NEST and began large-scale clinical verification.
Seoul National University Hospital has an infrastructure that produces and operates cell and gene therapy drugs for patients, so it can quickly perform actual patient sample-based evaluation. In addition, Quantamatrix has proven technology development and commercialization capabilities in the field of microorganism diagnosis, such as co-developing the technology uRAST (the world's first antibiotic susceptibility and pathogen identification within 13 hours without culture, published in Nature in 2024) with researchers at Seoul National University, Korea University, and Seoul National University Hospital. The two institutions plan to install two equipment within the advanced cell and gene therapy center of Seoul National University Hospital within this year, and conduct performance evaluation and technology advancement with actual clinical samples.
Beyond biopharmaceuticals, the technology developed in this study is expected to be used in various fields such as sepsis diagnosis, food safety tests, cosmetic aseptic verification, and early response to the spread of infectious diseases. Professor Kim Tae-hyun of Korea University said, `NEST has a great potential to expand to various industrial fields beyond simple research results,' adding, `We will increase reliability based on data accumulated in clinical settings.' ㅤ
Professor Kwon Sung-hoon of Seoul National University said, `We will actively continue further verification and clinical application so that this achievement can become a new criterion for ensuring patient safety"We plan to contribute to improving the global clinical and regulatory environment beyond Korea in the future."
|
This article was translated by Naver AI translator.