Development of an AI model that can predict osteoporosis status with chest X-rays
Nov 28, 2025
|
Chest X-rays contain bone structures close to fractures such as ribs, clavicles, and vertebrae, so if you use them, you can check osteoporosis early without a separate bone density test. In particular, the research team established an evaluation system that numerically verifies what bone structure AI actually judges based on, suggesting the possibility of reliable medical AI even in clinical practice.
Osteoporosis is a disease that increases the risk of fracture as the amount of bone decreases and the structure weakens, and the number of patients is increasing rapidly with aging. However, the standard test, DXA (bone density test), is often not sufficiently performed due to problems such as equipment accessibility. On the other hand, chest X-rays are already taken in most medical examinations, so if you can use them to evaluate osteoporosis together, it can be a new alternative to early diagnosis. However, AI models so far have had difficulties in clinical application due to the 'black box' problem in which the prediction process is not clearly explained.
Professor Park Sang-min of the Department of Family Medicine at Seoul National University Hospital (researcher Kim Jae-won) announced the results of a study comparing the predictive performance and explainability of various foundation-based AI models by analyzing data from 14,502 women who underwent chest X-rays and DXA tests between 2004 and 2019.
The research team first applied a foundation model pre-trained from various images to medical image analysis. The foundation model is an AI model trained with large-scale data, and fine-tuning it to fit medical images can achieve high performance even with limited medical data.
Four models were used in the study: the model trained with normal images (OpenCLIP, DINOv2) and the model trained with medical images (CheXagent, RAD-DINO), and a total of 12 AI models were created by applying three methods: ▲'linear verification' that newly learns only the last classification step' ▲'partial fine-tuning method' that re-learn only some layers of the model'LoRA method' that adds a low-dimensional matrix.
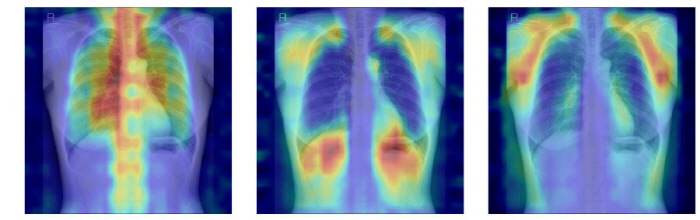
The AI extracts features around bone structures such as spine and ribs from the input chest X-rays and compares them with previously learned patterns to determine which state is most similar to that of normal (-T-score ≥ -1.0), osteopenia (-2.5 < T-score < -1.0), and osteoporosis (-2.5).
In addition, the research team designed a 'Explainability' evaluation system so that it can check which bones AI actually judges based on. We quantitatively verified whether AI is judged on the basis of clinically important bone structures by calculating how much AI's attention area, which is shown as Grad-CAM, matches the actual bone location by adding a specific bone part while covering all the bones.
As a result, the model applying LoRA method to the DINOv2 model showed the highest predictive performance with AUC 0.93 (95% CI 0.92-0.94). This model was evaluated as an optimal model with balanced predictive power and explainability due to its highest utilization of bone structure and excellent validity in the attention area.
It was also confirmed that medical imaging-based models are not always better, and high predictive performance does not improve explainability together. This shows that for medical AI to be utilized in real-world clinical practice, it is essential to verify the evidence for 'why it was judged that way' along with accuracy.
Researcher Kim Jae-won (Seoul National University Department of Medicine), the first author, explained, "When applying the foundation model to medical images, high performance alone is not sufficient, and a multidimensional evaluation system is needed to be trusted in the actual medical field. It is significant in that this study presented the criteria."
Corresponding author Professor Park Sang-min (Family Medicine Department) emphasized that "It is of great significance in that it presented a direction on how to select and utilize a foundation model by evaluating not only prediction accuracy but also whether AI's judgment basis can be transparently explained.".
Meanwhile, the results of this study were published in the recent issue of the international journal 『Osteophorosis International" in the field of osteoporosis and other metabolic bone diseases.
|
This article was translated by Naver AI translator.