Discovery of precision treatment targets that can inhibit liver fibrosis and inflammation at the same time...the possibility of developing the next generation of new drugs
Nov 26, 2025
|
With the recent rapid increase in the prevalence of obesity and metabolic diseases, the number of patients with early and moderate liver fibrosis based on metabolic inflammation is also steadily increasing. Liver fibrosis refers to an excessive accumulation of scar tissue (fibrotic tissue) in liver tissue as various liver diseases such as fatty liver, chronic viral hepatitis, alcoholic liver disease, and metabolic dysfunction-related steatohepatitis worsen.
Liver fibrosis in the early stages can be largely recovered in a form close to the normal liver if pathogenesis treatment such as weight loss, improved insulin resistance, and virus suppression is performed. However, if liver fibrosis persists and progresses to cirrhosis, it transforms into irreversible tissue and threatens life. Since there are no proven antifibrotic drugs and liver transplantation is the only treatment option, identifying therapeutic targets that act specifically in the fibrosis stage and developing targeted treatments based on them have been suggested as key goals of liver disease treatment strategies.
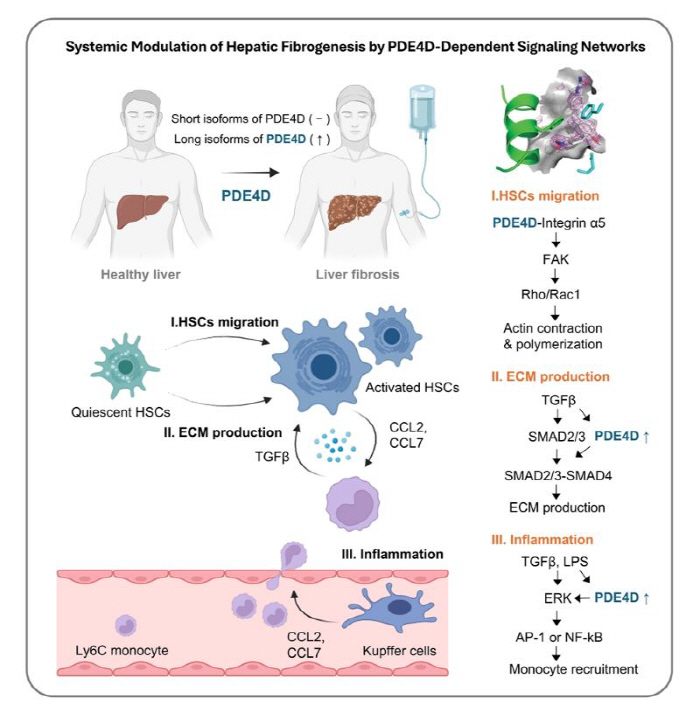
Professor Kim Jeong-han noted that a specific enzyme, PDE4D (phosphodiesterase 4D), is abnormally increased in the liver fibrosis stage through staging-specific transcriptome analysis of patients with liver disease. PDE4 family enzymes are enzymes that degrade intracellular cyclic adenosine monophosphate (cAMP) and have been noted as important regulatory factors in various inflammatory and fibrotic diseases. Previously, drugs that widely inhibit this PDE4-based enzyme have been effective in several animal experiments, but when administered to humans, side effects such as nausea and vomiting were severe, making it difficult to put them into practical use.
After a detailed analysis of individual isoforms in the PDE4 family, the research team found that among PDE4D, the form called 'long isoform' is particularly increased during liver fibrosis. 'Isoform' refers to a protein form that is made from the same gene but has slightly different functions.
In particular, this long isoform is greatly increased when hepatic cells, the key cells that cause liver fibrosis, are activated and has been found to act as a 'switch' that simultaneously promotes collagen production (fibrotic tissue formation), increased inflammatory signals, and cell migration. In other words, it has been identified that PDE4D long isoform is a key hub that simultaneously pushes inflammation and fibrosis of liver tissue. This is not just an approach that suppresses fibrosis, but it is significant that it has found a precise target that controls both fibrosis and inflammation.
Based on this, the research team proposed 'allosteric inhibitor', which selectively inhibits PDE4D long isoform, as a candidate for liver fibrosis treatment through a joint study with the U.S. National Institutes of Health and the Cedars-Sinai Medical Center. Allosteric inhibitors have the advantage of inhibiting selective and elaborate functions by inducing structural changes in proteins by binding to a specific regulatory site rather than an active site of the protein. It suggests the possibility of solving the side effects of existing pan-PDE4 inhibitors through a structural and electrostatic approach.
Professor Kim Jung-han said, `Although severe liver fibrosis is a very difficult disease to reverse, this study identified PDE4D, which increases selectively only in the fibrosis stage, as a new therapeutic target and demonstrated at the preclinical level that allosteric inhibitors aimed at it can simultaneously reduce fibrosis and inflammation"We will continue to develop the target and candidate materials derived this time to develop into precision medicine-based liver fibrosis treatments, leading to the next generation of antifibrotic drugs used in actual patient care." he said.
The study was conducted as an international joint study between Catholic University of Medicine and the U.S. National Institute of Health, and was supported by the Korea Research Foundation's Excellent Progressive Research, First Innovation Laboratory, and Basic Laboratory (BRL) Project, and was published in the latest issue of the prestigious international academic journal `Journal of Clinical Investigation" in the medical field.
|
This article was translated by Naver AI translator.