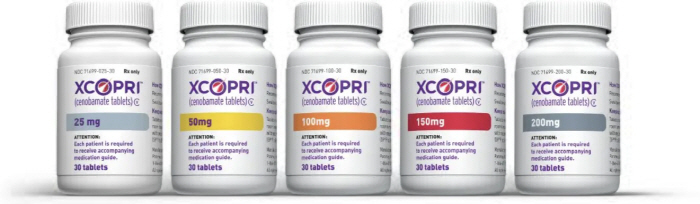
New drug approval innovation process No. 1 new drug is XCOPRIZE, a treatment for adult epilepsy patients
Nov 03, 2025
|
The Ministry of Food and Drug Safety said the drug is authorized as an additional therapy for partial seizure treatment with or without secondary systemic seizures in adult epilepsy patients, and expects to provide new treatment opportunities for epilepsy patients who are not properly controlled by the administration of existing anti-epilepsy drugs.
SK Biopharmaceuticals' own development 'Excoprijeong (Senovamate)' is the first item to be approved by applying the guidelines of the 'New Drug Product License and Review Business Procedure' enacted by the Ministry of Food and Drug Safety this year for rapid approval of new drugs. The Ministry of Food and Drug Safety formed a team dedicated to items (21 people) including experts in approval of new drugs ▲ Clinical Trials (GCP) and Manufacturing and Quality Management (GMP) Priority Review ▲ Customized face-to-face meetings (8 times) before and after applying for item approval.
The drug is an epilepsy treatment that normalizes the balance of nerve cells' excitatory and inhibitory signals by blocking sodium channels that transmit excitatory signals to the brain, and was approved for marketing by the U.S. Food and Drug Administration (FDA) in 2019, and the total number of cumulative prescription patients exceeded 140,000. However, as it was not introduced in Korea, there was a high demand for prompt introduction to relieve the inconvenience of patients prescribed abroad.
Meanwhile, Dong-A ST, which is in charge of licensing, sales, and production, received a transfer of finished drug (DP) production technology from SK Biopharmaceuticals in January last year to supply XCOPRIZE to 30 countries at home and abroad, and plans to expedite domestic insurance drug prices and registration.
This article was translated by Naver AI translator.