Demonstrate Renbatinib Excellence in Second-line Treatment of Hepatocellular Cancer...Confirmation of the extension of the survival period
Dec 01, 2025
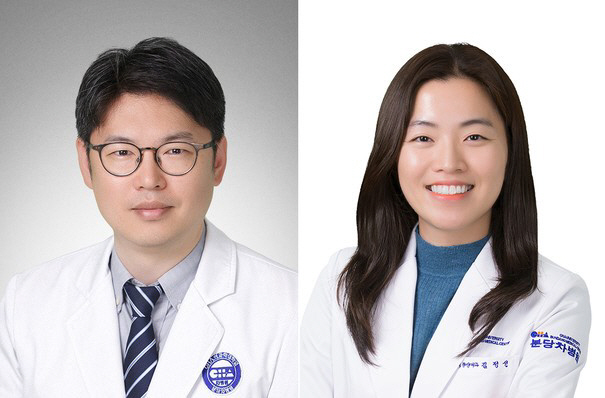
The research team led by Professor Jeon Hong-jae and Kim Jeong-sun of Cha Medical University Bundang Cha Hospital (Director Yoon Sang-wook) in Oncology Department at Cha Medical University (Director Yoon Sang-wook) has compared renvatinib or sorafenib as a second-line treatment strategy for patients with failed hepatocellular carcinoma (HCC) (hereinafter 'A+B'). This study presents an important clinical basis for optimizing hepatocellular carcinoma treatment strategies and is published in the latest issue of the international journal of hepatic biliary tract 'JHEP Reports (IF=7.5)'.
Hepatocellular carcinoma is a disease that ranks among the top causes of cancer death worldwide, with a five-year relative survival rate of only 39.4% in Korea, and the prognosis of advanced patients is still poor. The introduction of 'A+B' combination therapy in the first treatment dramatically extended the patient's survival period, but a significant number of patients have shown progression of the disease.
In particular, the optimal second-line treatment strategy that can be used after combination therapy of immuno-oncology drugs is not yet clear, so various targeted treatments (TKI) are used in actual clinical settings. Although it was thought that drugs targeting existing vascular endothelial growth factors (VEGFR) would have similar effects, actual studies have revealed that each drug has different effects, raising questions about existing assumptions.
The research team of professors Hong-jae Jeon and Jeong-sun Kim selected and analyzed 230 out of 1,210 patients who received A+B treatment from May 2018 to October 2024, of which 125 received renbatinib and 105 received sorafenib as second-line treatments. In order to minimize the difference in characteristics between the two treatment groups, the Propensity Score Matching (PSM) technique was applied to increase the objectivity of the comparison between the two groups.
As a result of a large clinical study, patients given renbatinib as a second-line treatment in hepatocellular carcinoma patients progressed after atezolizumab bevacizumab combination treatment recorded 5.5 months of progression-free survival (PFS) and 11.9 months of overall survival (OS), all significantly longer than the sorafenib-administered group (PFS 2.6 months, OS 7.4 months). The total survival period calculated from the start of the first-line treatment 'A+B' combination therapy was also 22.4 months for renbatinib and 14.3 months for sorafenib. The same trend was found in PSM analysis statistically tailored to patient characteristics, and renbatinib had a significantly higher proportion of patients who maintained the disease stably than sorafenib in the patient group who had no initial response to immuno-oncology drugs.
The results of this Leviathan study reverse the previous assumption that VEGFR-targeted treatment has equal effects and support that renbatinib is a more effective treatment option in second-line treatment after A+B treatment failure.
Professor Hongjae Jeon analyzed large multinational patient data to provide important basis for determining the order of treatment in real clinical practice, despite being an observational study"It is expected to mark an important turning point in improving survival and establishing treatment standards for patients with hepatocellular carcinoma in the future."
This study was conducted with the support of the mid-sized research support project of the Ministry of Science and ICT of the Korea Research Foundation.
Hepatocellular carcinoma is a disease that ranks among the top causes of cancer death worldwide, with a five-year relative survival rate of only 39.4% in Korea, and the prognosis of advanced patients is still poor. The introduction of 'A+B' combination therapy in the first treatment dramatically extended the patient's survival period, but a significant number of patients have shown progression of the disease.
In particular, the optimal second-line treatment strategy that can be used after combination therapy of immuno-oncology drugs is not yet clear, so various targeted treatments (TKI) are used in actual clinical settings. Although it was thought that drugs targeting existing vascular endothelial growth factors (VEGFR) would have similar effects, actual studies have revealed that each drug has different effects, raising questions about existing assumptions.
The research team of professors Hong-jae Jeon and Jeong-sun Kim selected and analyzed 230 out of 1,210 patients who received A+B treatment from May 2018 to October 2024, of which 125 received renbatinib and 105 received sorafenib as second-line treatments. In order to minimize the difference in characteristics between the two treatment groups, the Propensity Score Matching (PSM) technique was applied to increase the objectivity of the comparison between the two groups.
As a result of a large clinical study, patients given renbatinib as a second-line treatment in hepatocellular carcinoma patients progressed after atezolizumab bevacizumab combination treatment recorded 5.5 months of progression-free survival (PFS) and 11.9 months of overall survival (OS), all significantly longer than the sorafenib-administered group (PFS 2.6 months, OS 7.4 months). The total survival period calculated from the start of the first-line treatment 'A+B' combination therapy was also 22.4 months for renbatinib and 14.3 months for sorafenib. The same trend was found in PSM analysis statistically tailored to patient characteristics, and renbatinib had a significantly higher proportion of patients who maintained the disease stably than sorafenib in the patient group who had no initial response to immuno-oncology drugs.
The results of this Leviathan study reverse the previous assumption that VEGFR-targeted treatment has equal effects and support that renbatinib is a more effective treatment option in second-line treatment after A+B treatment failure.
Professor Hongjae Jeon analyzed large multinational patient data to provide important basis for determining the order of treatment in real clinical practice, despite being an observational study"It is expected to mark an important turning point in improving survival and establishing treatment standards for patients with hepatocellular carcinoma in the future."
This study was conducted with the support of the mid-sized research support project of the Ministry of Science and ICT of the Korea Research Foundation.
|
This article was translated by Naver AI translator.