A new treatment for ischemic cardiovascular disease is suggested...Development of direct cross-differentiation method for smooth muscle cells
Mar 26, 2025
|
Professor Yoon Young-seop and Dr. Chung Cho-romi of Yonsei University's Medical Life Sciences Department announced on the 26th that they have developed a new smooth muscle cell direct reprogramming method (direct cross-differentiation method) and confirmed the possibility of treating ischemic cardiovascular disease by inducing the production of new blood vessels in animal models of ischemic diseases.
The findings were published in the American Heart Association journal 'Circulation (IF 35.6)'.
Ischemic cardiovascular disease, one of the top 10 causes of death worldwide by the World Health Organization (WHO), is recognized as a major health problem due to its high mortality rate. Methods for managing and treating ischemic cardiovascular disease include medication, cardiovascular intervention, and vascular bypass surgery. However, there is no suitable treatment for patients who fail the procedure and surgery or cannot operate.
Although neovascularization using stem cell differentiation is attracting attention as an alternative treatment for these patients, this method has limitations in its application due to the characteristics of stem cells such as low differentiation rate, possibility of tumor development, and high production cost. Recently, direct reprogramming methods that overcome the limitations of stem cells are attracting new attention. Direct reprogramming is a method of inducing conversion into a desired cell by overexpressing a key transcription factor of a target cell into a somatic cell.
The research team developed a direct reprogramming method of vascular smooth muscle cells, one of the main cells that make up blood vessels for neovascularization. This has the advantage of being able to construct thicker and more stable blood vessels compared to the direct reprogramming method into human endothelial cells previously developed by the research team.
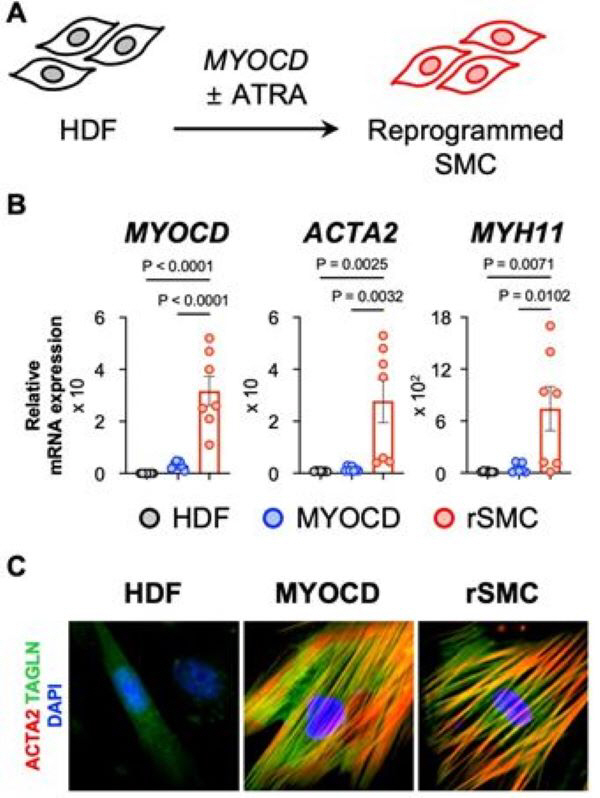
Myocardin, known as a key transcription factor of smooth muscle cells, and all-transretinoic acid, a metabolite of vitamin A, were combined to induce direct cross-differentiation from human dermal fibroblasts to smooth muscle cells.
As a result, smooth muscle cells' specific gene and protein expression were significantly increased in 'directly reprogrammed smooth muscle cells'(rSMCs), and smooth muscle cells' specific protein expression was additionally confirmed in immunostaining experiments and the cytoskeleton was clearly confirmed. The results of flow cytometry also confirmed that the expression rates of ACTA2 and MYH11, the major markers of smooth muscle cells, increased to 57.2±11.9% and 48.0±7.7%, respectively.
In addition, in order to evaluate the contractility of cells, one of the representative characteristics of smooth muscle cells, carbachol, a choline agent, was treated to confirm the degree of contraction of rSMCs and showed high contractility. Additionally, RNA-sequencing analysis also showed that specific gene expression of fibroblasts was inhibited in rSMCs, while contractility and specific gene expression of smooth muscle cells were significantly increased.
Additionally, the research team confirmed the new angiogenic ability and therapeutic effect of rSMCs using a lower limb ischemic mouse model.
Direct injection of rSMCs into lower extremity ischemia showed improved perfusion recovery and angiogenesis ability compared to other controls such as cell-free group and human dermal fibroblast group. We also confirmed the depression effect of some of the rSMCs entering the vessel wall and forming a new layer. The embedded rSMCs played the role of previously unknown microvascular main cells and contributed to the formation of vascular walls in capillaries, fibrillation, and arterioles.
Professor Young-seop Yoon confirmed the possibility of alternative treatment for patients with ischemic cardiovascular disease by confirming the viability of smooth muscle cells transplanted in vivo and the contribution of vascular composition through this study"We expect that the follow-up research will lead to tangible results in a wide range of medical fields such as therapeutic cell therapy products, vascular production through tissue engineering, and therapeutic drug development."
|
This article was translated by Naver AI translator.