Causes of Pancreatic Cancer Growth by Obesity, Fatty Acid Oxidation, Not Hormones
|
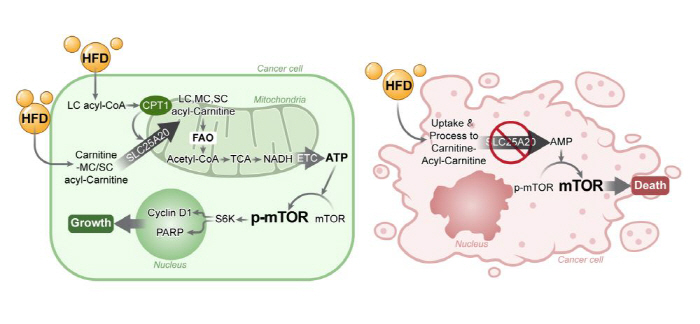
The results of this study were published in the May 2025 issue of the international journal `Theranostics' under the title 'Loss of SLC25A20 in pancreatic adenocarcinoma reversed the tumor-promoting effects of a high-fat diet.
Until now, the prevailing theory has been that obesity-induced tumor growth is due to indirect effects of inflammatory hormones such as Leptin, an inflammatory hormone, and insulin-like growth factor-1 (IGF-1) in liver and fat cells, but the research team proved that cancer cells produce ATP directly through Fatty Acid Oxidation (FAO) based on the theory that the energy metabolism of tumors depends on fatty acids (Kim Effect). This is evaluated as a complementary theory to the existing 'Warburg Effect', and it has been confirmed that a high-carb diet can inhibit cancer cell growth by up to 80% compared to a high-fat diet.
The research team observed that in a mouse pancreatic cancer model that provided a high-fat diet for 23 weeks, the weight doubled and the tumor size more than doubled compared to mice provided with the same calories as carbohydrates. In particular, when SLC25A20, a key gene that induces fatty acid oxidation, was genetically suppressed, the growth of cancer cells was suppressed to a level similar to that of normal diets, and in some cases, complete remission was observed in which tumor growth disappeared completely. In other words, we demonstrated that tumor growth effects by high-fat diets were completely reversed by regulating this gene. In addition, when the SLC25A20 gene was crossed with mice with defective SLC25A20 genes even in naturally occurring mouse pancreatic cancer (KPC model) with living immune cells, the overall survival rate was extended by an average of 7 weeks from 23 weeks to 30 weeks, demonstrating anticancer effects.
It is evaluated that this study presented a turning point in a new anticancer treatment strategy targeting fatty acid oxidation genes in cancer cells.
The research team explained that "SLC25A20 is a key channel for supplying fat to cancer cells, and if it is blocked, cancer cannot make energy properly.""With little side effects, it could be an optimal target for new chemotherapy.", he explained.
Meanwhile, the National Cancer Center is currently promoting the development and commercialization of new anticancer drugs targeting the SLC25A20 gene with its subsidiary NCC-Bio.
This article was translated by Naver AI translator.