Atypical EGFR Gene Mutant Non-Small Cell Lung Cancer Effect Confirmation
May 16, 2025
|
Professor Hong Min-hee of Yonsei Cancer Hospital's Lung Cancer Center announced on the 16th that laser-tinib, a third-generation EGFR-targeted treatment, with Professor Park Se-hoon of the Department of Hematologic Oncology at Samsung Medical Center, had an objective response rate of 50% for atypical EGFR mutations.
The findings were published in the Journal of Thoracic Oncology (IF 21.1).
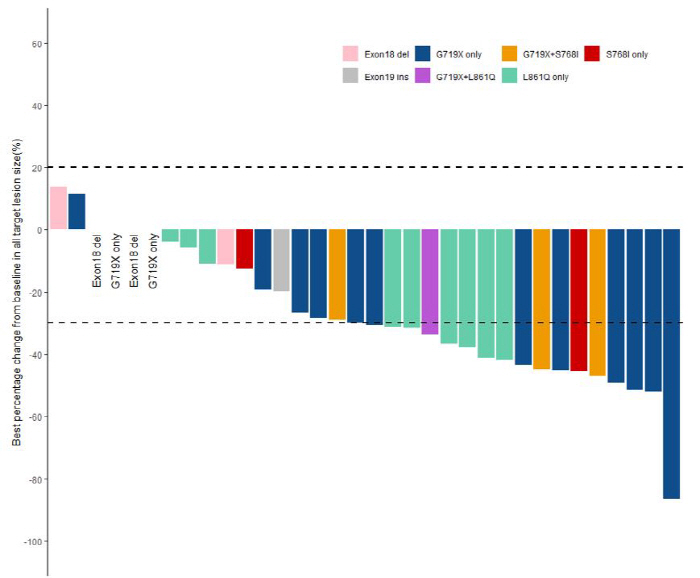
The EGFR gene mutation is a variant with a large number of Asians. Most are exon19 deletion or L858R variants, but approximately 10 to 20% are classified as atypical, such as G719X, L861Q, and S768I. They have a lower response rate to standard treatments than orthopedics and lack treatment options.
The research team conducted a clinical trial to evaluate the therapeutic effect of lasertinib, a third-generation EGFR-targeted treatment, on atypical patients. This clinical trial is a multicenter phase 2 study conducted by five hospitals in Korea, and 36 patients with atypical EGFR mutations without treatment history participated.
The objective response rate, which means more than 30% tumor reduction, and the disease control rate, which is a combination of tumor reduction and patients whose tumors do not grow, reached 50% and 88.9%, respectively. The response rate of mutations such as G719X, L861Q, and S768I, which showed 70-80% of atypical patients, was 54.8%. In particular, the response rate exhibited by G719X single-variant patients, which account for the most type among clinical participants, was 61%, and the median progression-free survival period was 20.3 months.
The side effects of the patient were not at the level of concern. 33.3% of patients showed grade 3 or higher among grade 1 to 5 side effects classified by the National Cancer Institute, but it was manageable without drug loss or interruption.
In addition, the research team explored the mechanism of laser tinib resistance through blood tests before and after treatment, and some patients showed genetic mutations such as APC, TP53, RET, and ERBB2, not EGFR, suggesting clues that could help make subsequent treatment decisions.
Professor Hong Min-hee explained, "This study is a prospective study that showed that laser-tinib, a third-generation EGFR-targeted treatment, can be a practical treatment alternative for patients with atypical EGFR-mutated lung cancer with limited treatment options. We are planning a follow-up study to find ways to improve treatment outcomes, such as laser-tinib monotherapy as well as combination therapy with other treatments."
|
This article was translated by Naver AI translator.