Expansion of GLP1 family obesity treatment injection market to Hugo Beyer Mountain...Ministry of Food and Drug Safety advises on specialty drugs used for patients with high obesity
Aug 25, 2025
|
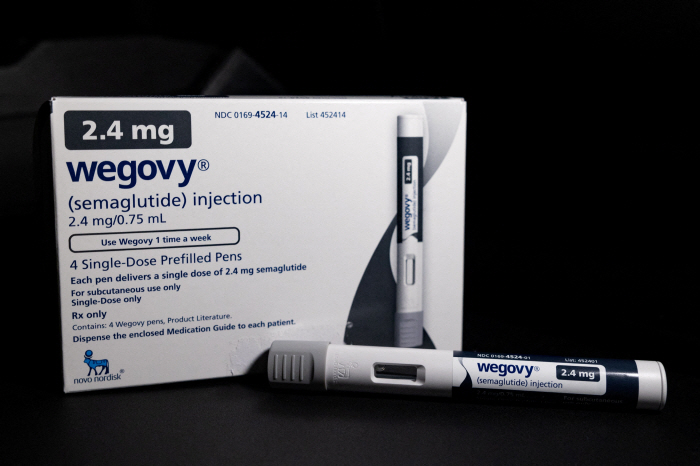
Following Novo Nordisk's Hugo Bee, Eli Lilly's Mountain Jaro has recently been released in Korea, and the size of the injection-type obesity treatment market is exploding
According to a report by Hana Securities, IQVIA, a pharmaceutical research firm, analyzed that the domestic obesity drug market grew 162.3% in the first quarter of this year to 108.6 billion won compared to the same period last year (41.4 billion won). It is evaluated that sales of Hugo Bee recorded KRW 79.4 billion, driving the obesity drug market. According to Korea Lily, the size of the domestic obesity drug market in the second quarter was 168.6 billion won, up 60 billion won from the first quarter. Citing IQVIA data, Lilly Korea said that in the second quarter, the GLP-1 treatment market, such as Saxenda, Hugobee, and Mountaineer, reached about 141.6 billion won in the obesity drug market. The prevailing analysis is that the size of the domestic obesity drug market could grow as competition is expected to heat up further due to the launch of Maunza in Korea.
In this regard, the Ministry of Food and Drug Safety announced on the 25th that only patients with obesity should be used carefully according to the prescription of medical experts.
GLP-1 series obesity drug injections are prescribed to adult obese patients with an initial body mass index (BMI) of 30kg/m2 or more, or adult overweight patients with more than 27kg/m2 or less than 30kg/m2 and one or more weight-related comorbidities such as hypertension.
According to clinical trial results, gastrointestinal adverse reactions (nausea, vomiting, diarrhea, constipation, etc.) and injection site reactions (rashes, pain, swelling, etc.) are common even if the obesity treatment is used within the permitted range, and side effects such as hypersensitivity, hypoglycemia, acute pancreatitis, cholelithiasis, and fluid reduction may occur.
In addition, some drugs are prohibited from administration if they have underlying diseases such as thyroid medullary cancer, so be sure to consult with a specialist, and patients with a related medical history should be administered especially carefully because hypoglycemia and retinopathy may occur in diabetic patients (type 2).
According to the Ministry of Food and Drug Safety, "An obesity treatment must be used in accordance with the pharmacist's preparation and medication guidance after a doctor's prescription, and should not be distributed or purchased through overseas direct purchase or personal sales online."
The Ministry of Food and Drug Safety plans to designate GLP-1-based obesity treatment injections as intensive monitoring targets to continuously monitor side effects with the Korea Institute of Drug Safety and to focus on illegal sales and advertising of obesity treatment on online platforms and social media. In addition, in order to promote the proper use of obesity drugs, we plan to publish a guide (leaflet) containing ▲ diseases using obesity drugs with the Korea Institute of Drug Safety ▲ correct administration method ▲ storage and disposal method ▲ precautions when administering ▲ abnormal reaction (side effect) reporting method.
This article was translated by Naver AI translator.