Seoul National University Hospital Suggests Early Diagnosis of Childhood Moyamoya Disease with Blood Test
Aug 01, 2025
|
The research team analyzed the patient's blood and found that the level of specific microRNA (miRNA) called 'miR-512-3p' was significantly higher in moyamoya disease patients than in the control group. This biomarker is closely associated with abnormal angiogenesis and shows an important potential as an early diagnosis and therapeutic target for Moyamoya's disease.
A research team led by Professor Kim Seung-ki of Pediatric Neurosurgery at Seoul National University Hospital, Dr. Ko Eun-jung of JLK, and Professor Choi Seung-ah of the Pediatric Cancer and Rare Disease Support Project announced on the 31st that they discovered a new biomarker called miR-512-3p in the plasma of pediatric Moyamoya disease patients and announced the results of the study on the 31st that identified its function and mechanism of action.
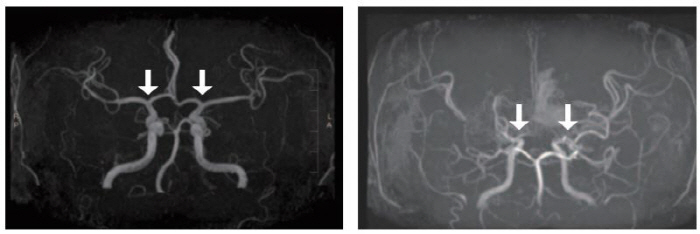
Moyamoya disease is a chronic progressive cerebrovascular disease in which the blood vessels gradually narrow in the branching area of the internal artery that supplies blood to the cerebrum without any specific cause. As a result, blood flow becomes insufficient and abnormal microvessels are grown to make up for insufficient blood flow, but these blood vessels do not supply enough blood and are prone to bursting, causing serious childhood strokes such as cerebral infarction and cerebral hemorrhage.
Until now, invasive cerebrovascular angiography has been a standard test to confirm Moyamoya's disease, which has been particularly burdensome for children. Although there are also non-invasive tests such as MRI/A, vascular stenosis is sometimes exaggerated and moyamoya blood vessels at the base of the brain are difficult to assess in detail, so early diagnosis and disease progression were limited. Accordingly, the need for liquid biopsy (diagnosis through body fluids such as blood) has been steadily raised.
The research team analyzed extracellular vesicles (EV) in the blood of 23 patients with moyamoya disease and 13 healthy controls and found a biomarker called miR-512-3p. Extracellular vesicles are small particles released from cells, containing genetic information such as miRNA, and play an important role in intercellular signaling and mass transmission.
As a result of our study, the expression level of miR-512-3p was statistically significantly higher in Moyamoya disease patients compared to controls (p=0.0397). In addition, it was confirmed that miR-512-3p acts on the RHOA signaling system, which is an vascular formation regulatory pathway, inhibiting the expression of the 'ARHGEF3' gene, thereby interfering with angiogenesis. In other words, the research team found that miR-512-3p plays a key role in causing abnormal vascular networks through the RHOA pathway.
Additionally, the team confirmed whether the expression of ARHGEF3 is restored and the vascular formation function is improved by inhibiting the function of miR-512-3p. As a result of suppressing miR-512-3p in the experiment, it was confirmed that GTPase activity increased by 2.3 times, and vascular formation ability was improved by 1.7 times in Endothelioid Forming Cells (ECFCs). Increased GTPase activity implies activation of important signaling pathways that promote cell migration and vascular formation. This suggests that miR-512-3p may play an important role not only as a diagnostic marker for moyamoya disease, but also as a therapeutic target.
The diagnostic accuracy of miR-512-3p was evaluated as AUC 0.82, indicating that miR-512-3p is a biomarker with good accuracy for the diagnosis of moyamoya disease. The research team said the study marks an important first step toward early diagnosis and customized treatment of moyamoya disease, and it is expected that pediatric patients will be able to be diagnosed with the disease in a non-invasive way and receive help in treatment in the future.
Professor Kim Seung-ki (Pediatric Neurosurgery) of Seoul National University Hospital said, `This study is an important study that proved that early diagnosis of Moya moya disease is possible through blood tests.' `We hope that using this biomarker, pediatric patients can be diagnosed with the disease early and receive customized treatment.'
Meanwhile, the study was carried out with the support of the Korea Research Foundation, the Seoul National University Hospital Research Fund, and the Lee Kun-hee Children's Cancer and Rare Diseases Overcoming Project, and the results of the study were published in the latest issue of the international journal 『Scientific Reports』. In addition, the results of this study are a technology for diagnosing moyamoya disease through miR-512-3p expression levels and a treatment screening method using the 'ARHGEF3' gene, and domestic patent registration has been completed.
|
This article was translated by Naver AI translator.