Accelerating global targeting of the domestic diagnostic industry...SD BioSensor obtains Fast European Certification for simultaneous diagnosis and unveils the Seagen Unmanned PCR Automation System Society
Aug 06, 2025
|
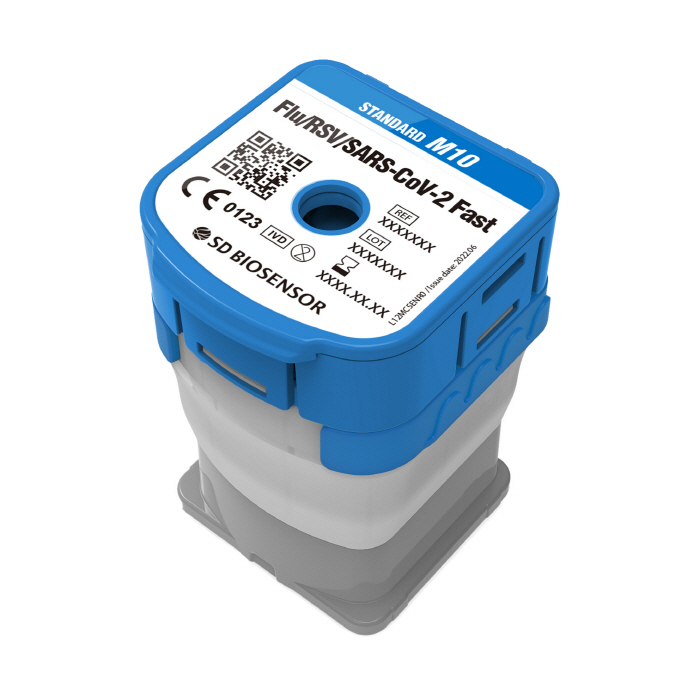
SD Biosensor, a company specializing in in vitro diagnostics, announced on the 6th that it has obtained European In vitro Diagnostic Medical Device Regulations (hereinafter referred to as 'CE-IVDR NPT') for on-site molecular diagnosis 'Standard M10 Flu/RSV/SARS-CoV-2 Fast (hereinafter referred to as M10 FRS Fast).'
M10 FRS Fast, which can simultaneously identify three major respiratory diseases, flu, respiratory syncytial virus, and COVID-19, can detect a total of nine genes within 25 to 36 minutes, and targets two or more genes for each pathogen for more comprehensive and precise diagnosis. The CE-IVDR Near-Patient Testing (NPT) certification obtained this time targets on-site diagnostic products used in patient proximity care environments, unlike common CE-IVDRs, and stricter certification standards are applied. Through this certification, M10 FRS Fast will meet the demanding standards of the European field diagnosis market to prove the reliability of the product and target the market based on the speed, accuracy, and excellent ease of use in the actual clinical field.
"M10 FRS Fast is a very competitive product in the European market that requires both speed and accuracy as it can simultaneously identify three major respiratory diseases in 25 minutes," an SD biosensor official said. "In particular, obtaining CE-IVDR NPT certification ahead of the respiratory disease epidemic in Europe is a very timely achievement and is expected to be a catalyst for the market expansion of the field molecular diagnostic platform M10 as well as the actual sales expansion in the future." he said. "This certification enables immediate product introduction in various medical environments in Europe, as well as specific market expansion such as public procurement bidding and government quarantine projects."We plan to use this as a stepping stone to accelerate the expansion of global share, including Europe" he added.
|
Chun Jong-yoon, chairman of Seegene, predicted that unmanned automation and data-based detailed inspection will be the key to leading the paradigm of molecular diagnosis in the future. It is also determined to open a new era for the diagnostic industry, just as smartphones and electric vehicles have changed the landscape of the mobile phone and automobile industries"The background of the development was explained. "Now, a single test result is not enough, and accurate judgment is possible only when statistics and similar case-based information are added "STAgora™ will provide critical insight to medical staff by collecting, analyzing, and sharing infectious disease data in real time, not only inside hospitals, but also at the national level, and even around the world." In addition, STAGORA™ evaluated that "△automatic statistical processing △ reliability of test results △ search for similar patient cases, etc."
This article was translated by Naver AI translator.