Genetic hearing loss-causing mutation proves hearing recovery with one genetic correction
Aug 25, 2025
|
A joint research team consisting of Lee Sang-yeon, a professor of pediatric otolaryngology at Seoul National University Hospital, and Bae Sang-soo, a professor of biochemistry at Seoul National University (Jeong So-hyang's brain science cooperation course and Han Je-sol's tumor biology cooperation course) announced on the 25th that they have developed a humanized mouse model with mutations in the MPZL2 gene that causes hereditary hearing loss and applied their own genetic scissors to confirm hearing recovery and hearing cell recovery.
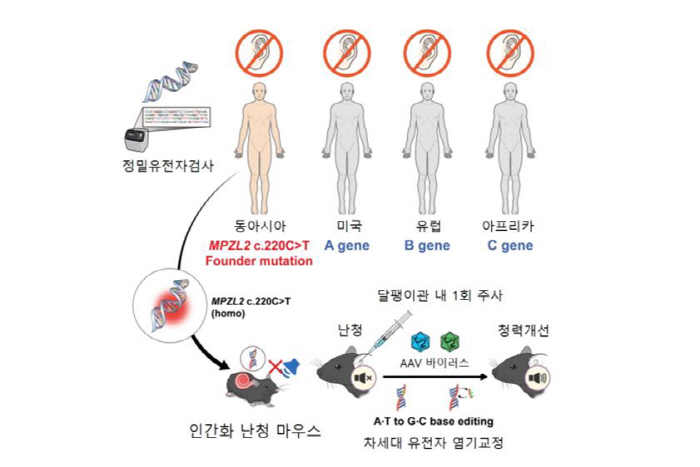
The c.220C>T variation of the MPZL2 gene is one of the leading causes of DFNB111 type sensory neurogenic hearing loss, especially in East Asian populations, occurring at a high frequency and observed in approximately 10% of all patients with hereditary hearing loss. This mutation is a nonsense mutation in which the CAG codon, which encodes glutamine, turns into TAG, a termination signal, and as MPZL2 protein production stops, it causes rapid hearing loss after adolescence and gradually progresses to high hearing loss. However, to date, there has been no fundamental way to treat this.
The research team analyzed a genetic hearing loss cohort consisting of 1,437 domestic and foreign households and c.It was confirmed that the 220C>T mutation was the main cause, and a humanized mouse model was produced that simulated it. Subsequently, the latest adenine base correction gene scissors (ABE8eWQ-SpRY) with increased calibration efficiency and accuracy were developed on their own, and a strategy was established to accurately correct the mutant base with a normal sequence. This gene scissors works by converting adenine (A) into guanine (G) without cutting double-stranded DNA, which is a next-generation gene editing technology with low cell damage and high target accuracy.
The research team loaded the gene scissors into AAV-ie, a modified vector of adenos-associated virus (AAV), and delivered them to the ear by injecting them only once through the mouse's round window. AAV-ie has high delivery efficiency to auditory cells and works stably, and the gene scissors correct the disease-causing adenine base to normal guanine by accurately reaching the mutant site under the guidance of guide RNA (sgRNA).
The therapeutic effect was measured objectively through auditory brain stem induction reaction (ABR) and diverticulum radiation (DPOAE) tests. As a result, hearing improvement of 20 to 30 dB in the entire frequency band was confirmed in the treatment group, and this effect lasted for more than 20 weeks. This is evaluated as an important result supporting the in vivo applicability and treatment efficiency of endothelial gene correction techniques.
In tissue analysis, the survival rate of ectophagocytes (OHC), which are auditory cells, and supporting cells (DC) that support them increased significantly, and the tissue structure of the cochlear duct was also clearly restored. This suggests that gene correction substantially contributes not only to the function of auditory cells but also to histological restoration.
The research team used the Cas-OFFinder program at the DNA level to predict the possibility of off-target correction to evaluate the accuracy and safety of treatment, and verified that there was no off-target through GUIDE-seq analysis. As a result of analyzing the occurrence of correction in areas other than the target by actual cochlear tissue sequencing analysis, it was confirmed that there was no effect of deviation from the target and no off-target. RNA-seq analysis was also performed at the RNA level to confirm that non-target RNA editing did not occur, proving that the gene scissors used this time had high target accuracy and in vivo safety at the same time.
Professor Bae Sang-soo of Seoul National University of Medicine (Biochemistry Class) said "This study is meaningful in that it substantially proved the possibility of precision gene therapy by correcting genetic hearing loss mutations that are highly frequent in East Asian populations with self-developed genetic scissors.".
Professor Lee Sang-yeon of Seoul National University Hospital (Children's Eye Ophthalmology) "In the past, hearing loss treatment was limited to hearing aids and artificial wow, but we expect that it will open a new way for customized gene therapy to patients with clear genetic causes."We will strive to lead to actual patient treatment through additional preclinical and clinical studies."
On the other hand, the study was carried out with the support of the Seoul Medical University Medical School's Medical Scientist Project, Lee Kun-hee's pediatric cancer and rare disease research project, the Korea Research Foundation's excellent new research, and the Seoul-type bio project. The results of the study were published in the latest issue of Nature Communications (IF 15.7), one of the best international academic journals.
|
This article was translated by Naver AI translator.